β-Galactosylceramidase Deficiency Causes Upregulation of Long Pentraxin-3 in the Central Nervous System of Krabbe Patients and Twitcher Mice
Abstract
:1. Introduction
2. Results
2.1. PTX3 Immunoreactivity in the Brain of Krabbe Patients
2.2. Ptx3 Upregulation in Twitcher CNS
2.3. PTX3 Overexpression Reduces Clinical Symptoms and Spinal Cord Inflammation in Twitcher Mice
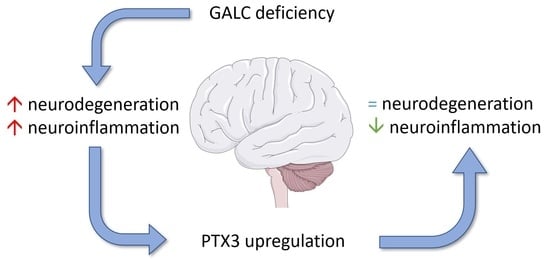
3. Discussion
4. Materials and Methods
4.1. Histopathology of Human GLD Biopsies
4.2. Animals
4.3. Quantitative RT-PCR Analysis
4.4. Immunohistochemical Analysis
4.5. Western Blotting
4.6. Serum PTX3
4.7. Assessment of Animal Clinical Features
4.8. Psychosine Extraction and MS Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, K.; Suzuki, Y. Globoid cell leucodystrophy (Krabbe’s disease): Deficiency of galactocerebroside beta-galactosidase. Proc. Natl. Acad. Sci. USA 1970, 66, 302–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballabio, A.; Gieselmann, V. Lysosomal disorders: From storage to cellular damage. Biochim. Biophys. Acta 2009, 1793, 684–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K. Twenty five years of the “psychosine hypothesis”: A personal perspective of its history and present status. Neurochem. Res. 1998, 23, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Igisu, H.; Suzuki, K. Progressive accumulation of toxic metabolite in a genetic leukodystrophy. Science 1984, 224, 753–755. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Benitez, B.A.; Nagree, M.S.; Dearborn, J.T.; Jiang, X.; Guzman, M.A.; Woloszynek, J.C.; Giaramita, A.; Yip, B.K.; et al. Genetic ablation of acid ceramidase in Krabbe disease confirms the psychosine hypothesis and identifies a new therapeutic target. Proc. Natl. Acad. Sci. USA 2019, 116, 20097–20103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenger, D.A.; Rafi, M.A.; Luzi, P.; Datto, J.; Costantino-Ceccarini, E. Krabbe disease: Genetic aspects and progress toward therapy. Mol. Genet. Metab. 2000, 70, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Nakamura, S.; Momoi, M.; Yamaji, T.; Takematsu, H.; Yano, H.; Sabe, H.; Yamamoto, A.; Kawasaki, T.; Kozutsumi, Y. Inhibition of cytokinesis by a lipid metabolite, psychosine. J. Cell Biol. 2000, 149, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Kozutsumi, Y.; Kanazawa, T.; Sun, Y.; Yamaji, T.; Yamamoto, H.; Takematsu, H. Sphingolipids involved in the induction of multinuclear cell formation. Biochim. Biophys. Acta 2002, 1582, 138–143. [Google Scholar] [CrossRef]
- Nicaise, A.M.; Bongarzone, E.R.; Crocker, S.J. A microglial hypothesis of globoid cell leukodystrophy pathology. J. Neurosci. Res. 2016, 94, 1049–1061. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K. Globoid cell leukodystrophy (Krabbe’s disease): Update. J. Child Neurol. 2003, 18, 595–603. [Google Scholar] [CrossRef]
- Loonen, M.C.; Van Diggelen, O.P.; Janse, H.C.; Kleijer, W.J.; Arts, W.F. Late-onset globoid cell leucodystrophy (Krabbe’s disease). Clinical and genetic delineation of two forms and their relation to the early-infantile form. Neuropediatrics 1985, 16, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.H.; Graham, S.C.; Read, R.J.; Deane, J.E. Structural snapshots illustrate the catalytic cycle of beta-galactocerebrosidase, the defective enzyme in Krabbe disease. Proc. Natl. Acad. Sci. USA 2013, 110, 20479–20484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escolar, M.L.; Poe, M.D.; Provenzale, J.M.; Richards, K.C.; Allison, J.; Wood, S.; Wenger, D.A.; Pietryga, D.; Wall, D.; Champagne, M.; et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N. Engl. J. Med. 2005, 352, 2069–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garlanda, C.; Bottazzi, B.; Bastone, A.; Mantovani, A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu. Rev. Immunol. 2005, 23, 337–366. [Google Scholar] [CrossRef]
- Fornai, F.; Carrizzo, A.; Ferrucci, M.; Damato, A.; Biagioni, F.; Gaglione, A.; Puca, A.A.; Vecchione, C. Brain diseases and tumorigenesis: The good and bad cops of pentraxin3. Int. J. Biochem. Cell Biol. 2015, 69, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Polentarutti, N.; Bottazzi, B.; Di Santo, E.; Blasi, E.; Agnello, D.; Ghezzi, P.; Introna, M.; Bartfai, T.; Richards, G.; Mantovani, A. Inducible expression of the long pentraxin PTX3 in the central nervous system. J. Neuroimmunol. 2000, 106, 87–94. [Google Scholar] [CrossRef]
- Ravizza, T.; Moneta, D.; Bottazzi, B.; Peri, G.; Garlanda, C.; Hirsch, E.; Richards, G.J.; Mantovani, A.; Vezzani, A. Dynamic induction of the long pentraxin PTX3 in the CNS after limbic seizures: Evidence for a protective role in seizure-induced neurodegeneration. Neuroscience 2001, 105, 43–53. [Google Scholar] [CrossRef]
- Rodriguez-Grande, B.; Varghese, L.; Molina-Holgado, F.; Rajkovic, O.; Garlanda, C.; Denes, A.; Pinteaux, E. Pentraxin 3 mediates neurogenesis and angiogenesis after cerebral ischaemia. J. Neuroinflammation 2015, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Grande, B.; Swana, M.; Nguyen, L.; Englezou, P.; Maysami, S.; Allan, S.M.; Rothwell, N.J.; Garlanda, C.; Denes, A.; Pinteaux, E. The acute-phase protein PTX3 is an essential mediator of glial scar formation and resolution of brain edema after ischemic injury. J. Cereb. Blood Flow Metab. 2014, 34, 480–488. [Google Scholar] [CrossRef]
- Ryu, W.S.; Kim, C.K.; Kim, B.J.; Kim, C.; Lee, S.H.; Yoon, B.W. Pentraxin 3: A novel and independent prognostic marker in ischemic stroke. Atherosclerosis 2012, 220, 581–586. [Google Scholar] [CrossRef]
- Lee, H.W.; Choi, J.; Suk, K. Increases of pentraxin 3 plasma levels in patients with Parkinson’s disease. Mov. Disord. 2011, 26, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Yamanaka, T.; Jacobs, J.M.; Teixeira, F.; Suzuki, K. The Twitcher mouse: An enzymatically authentic model of human globoid cell leukodystrophy (Krabbe disease). Brain Res. 1980, 202, 479–483. [Google Scholar] [CrossRef]
- Suzuki, K. The twitcher mouse: A model for Krabbe disease and for experimental therapies. Brain Pathol. 1995, 5, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Lee, S.; Lee, W.H.; Suk, K. Analysis of glial secretome: The long pentraxin PTX3 modulates phagocytic activity of microglia. J. Neuroimmunol. 2010, 229, 63–72. [Google Scholar] [CrossRef]
- Suzuki, K. The twitcher mouse. A model of human globoid cell leukodystrophy (krabbe’s disease). Am. J. Pathol. 1983, 111, 394–397. [Google Scholar]
- Belleri, M.; Ronca, R.; Coltrini, D.; Nico, B.; Ribatti, D.; Poliani, P.L.; Giacomini, A.; Alessi, P.; Marchesini, S.; Santos, M.B.; et al. Inhibition of angiogenesis by beta-galactosylceramidase deficiency in globoid cell leukodystrophy. Brain 2013, 136, 2859–2875. [Google Scholar] [CrossRef] [Green Version]
- Ko, C.Y.; Chang, L.H.; Lee, Y.C.; Sterneck, E.; Cheng, C.P.; Chen, S.H.; Huang, A.M.; Tseng, J.T.; Wang, J.M. CCAAT/enhancer binding protein delta (CEBPD) elevating PTX3 expression inhibits macrophage-mediated phagocytosis of dying neuron cells. Neurobiol. Aging 2012, 33, 422.e411–422.e425. [Google Scholar] [CrossRef]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Claycomb, K.I.; Winokur, P.N.; Johnson, K.M.; Nicaise, A.M.; Giampetruzzi, A.W.; Sacino, A.V.; Snyder, E.Y.; Barbarese, E.; Bongarzone, E.R.; Crocker, S.J. Aberrant production of tenascin-C in globoid cell leukodystrophy alters psychosine-induced microglial functions. J. Neuropathol. Exp. Neurol. 2014, 73, 964–974. [Google Scholar] [CrossRef] [Green Version]
- Yao, K.; Zu, H.B. Microglial polarization: Novel therapeutic mechanism against Alzheimer’s disease. Inflammopharmacology 2020, 28, 95–110. [Google Scholar] [CrossRef]
- Hill, C.H.; Cook, G.M.; Spratley, S.J.; Fawke, S.; Graham, S.C.; Deane, J.E. The mechanism of glycosphingolipid degradation revealed by a GALC-SapA complex structure. Nat. Commun. 2018, 9, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Shinoda, H.; Goto, I.; Yamanaka, T.; Suzuki, Y. Globoid cell leukodystrophy is a generalized galactosylsphingosine (psychosine) storage disease. Biochem. Biophys. Res. Commun. 1987, 144, 41–46. [Google Scholar] [CrossRef]
- Whitfield, P.D.; Sharp, P.C.; Taylor, R.; Meikle, P. Quantification of galactosylsphingosine in the twitcher mouse using electrospray ionization-tandem mass spectrometry. J. Lipid Res. 2001, 42, 2092–2095. [Google Scholar] [CrossRef]
- Ronca, R.; Giacomini, A.; Di Salle, E.; Coltrini, D.; Pagano, K.; Ragona, L.; Matarazzo, S.; Rezzola, S.; Maiolo, D.; Torrella, R.; et al. Long-Pentraxin 3 Derivative as a Small-Molecule FGF Trap for Cancer Therapy. Cancer Cell 2015, 28, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Oggioni, M.; Mercurio, D.; Minuta, D.; Fumagalli, S.; Popiolek-Barczyk, K.; Sironi, M.; Ciechanowska, A.; Ippati, S.; De Blasio, D.; Perego, C.; et al. Long pentraxin PTX3 is upregulated systemically and centrally after experimental neurotrauma, but its depletion leaves unaltered sensorimotor deficits or histopathology. Sci. Rep. 2021, 11, 9616. [Google Scholar] [CrossRef]
- Ummenthum, K.; Peferoen, L.A.; Finardi, A.; Baker, D.; Pryce, G.; Mantovani, A.; Bsibsi, M.; Bottazzi, B.; Peferoen-Baert, R.; van der Valk, P.; et al. Pentraxin-3 is upregulated in the central nervous system during MS and EAE, but does not modulate experimental neurological disease. Eur. J. Immunol. 2016, 46, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Daigo, K.; Inforzato, A.; Barajon, I.; Garlanda, C.; Bottazzi, B.; Meri, S.; Mantovani, A. Pentraxins in the activation and regulation of innate immunity. Immunol. Rev. 2016, 274, 202–217. [Google Scholar] [CrossRef] [Green Version]
- Park, H.W.; Moon, H.E.; Kim, H.S.; Paek, S.L.; Kim, Y.; Chang, J.W.; Yang, Y.S.; Kim, K.; Oh, W.; Hwang, J.H.; et al. Human umbilical cord blood-derived mesenchymal stem cells improve functional recovery through thrombospondin1, pantraxin3, and vascular endothelial growth factor in the ischemic rat brain. J. Neurosci. Res. 2015, 93, 1814–1825. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, H.; Zheng, J.F.; Guo, Z.D.; Huang, Z.J.; Wu, Y.; Zhong, J.J.; Sun, X.C.; Cheng, C.J. Pentraxin 3 contributes to neurogenesis after traumatic brain injury in mice. Neural Regen. Res. 2020, 15, 2318–2326. [Google Scholar]
- Lian, C.; Huang, Q.; Zhong, X.; He, Z.; Liu, B.; Zeng, H.; Xu, N.; Yang, Z.; Liao, C.; Fu, Z.; et al. Pentraxin 3 secreted by human adipose-derived stem cells promotes dopaminergic neuron repair in Parkinson’s disease via the inhibition of apoptosis. FASEB J. 2021, 35, e21748. [Google Scholar] [CrossRef]
- Meisingset, T.W.; Ricca, A.; Neri, M.; Sonnewald, U.; Gritti, A. Region- and age-dependent alterations of glial-neuronal metabolic interactions correlate with CNS pathology in a mouse model of globoid cell leukodystrophy. J. Cereb. Blood Flow Metab. 2013, 33, 1127–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ida, H.; Rennert, O.M.; Watabe, K.; Eto, Y.; Maekawa, K. Pathological and biochemical studies of fetal Krabbe disease. Brain Dev. 1994, 16, 480–484. [Google Scholar] [CrossRef]
- Martin, J.J.; Leroy, J.G.; Ceuterick, C.; Libert, J.; Dodinval, P.; Martin, L. Fetal Krabbe leukodystrophy. A morphologic study of two cases. Acta Neuropathol. 1981, 53, 87–91. [Google Scholar] [CrossRef]
- Schlaeger, T.M.; Bartunkova, S.; Lawitts, J.A.; Teichmann, G.; Risau, W.; Deutsch, U.; Sato, T.N. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc. Natl. Acad. Sci. USA 1997, 94, 3058–3063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salustri, A.; Garlanda, C.; Hirsch, E.; De Acetis, M.; Maccagno, A.; Bottazzi, B.; Doni, A.; Bastone, A.; Mantovani, G.; Beck Peccoz, P.; et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 2004, 131, 1577–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guenzel, A.J.; Turgeon, C.T.; Nickander, K.K.; White, A.L.; Peck, D.S.; Pino, G.B.; Studinski, A.L.; Prasad, V.K.; Kurtzberg, J.; Escolar, M.L.; et al. The critical role of psychosine in screening, diagnosis, and monitoring of Krabbe disease. Genet. Med. 2020, 22, 1108–1118. [Google Scholar] [CrossRef]
- Sakai, N.; Inui, K.; Tatsumi, N.; Fukushima, H.; Nishigaki, T.; Taniike, M.; Nishimoto, J.; Tsukamoto, H.; Yanagihara, I.; Ozono, K.; et al. Molecular cloning and expression of cDNA for murine galactocerebrosidase and mutation analysis of the twitcher mouse, a model of Krabbe’s disease. J. Neurochem. 1996, 66, 1118–1124. [Google Scholar] [CrossRef]
- Marmiroli, P.; Rodriguez-Menendez, V.; Rigamonti, L.; Tonoli, E.; Rigolio, R.; Cavaletti, G.; Tredici, G.; Vercelli, A. Neuropathological changes in the peripheral nervous system and spinal cord in a transgenic mouse model of Niemann-Pick disease type A. Clin. Neuropathol. 2009, 28, 263–274. [Google Scholar]
- Shen, J.S.; Watabe, K.; Ohashi, T.; Eto, Y. Intraventricular administration of recombinant adenovirus to neonatal twitcher mouse leads to clinicopathological improvements. Gene Ther. 2001, 8, 1081–1087. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.T.; Li, S.C.; Buck, W.R.; Haskins, M.E.; Wu, S.W.; Khoo, K.H.; Sidransky, E.; Bunnell, B.A. Selective extraction and effective separation of galactosylsphingosine (psychosine) and glucosylsphingosine from other glycosphingolipids in pathological tissue samples. Neurochem. Res. 2011, 36, 1612–1622. [Google Scholar] [CrossRef] [Green Version]







| Deidentified Case ID | * Pathology | § GFAP+ | § PTX3+ |
|---|---|---|---|
| CW16 064 | 3 (severe) | 2 (gliosis) | 2 (mainly in perivascular globoid cells) |
| CW17 060 | 3 (severe) | 2 (gliosis) | 2 (mainly in perivascular globoid cells) |
| CW18 060 | 3 (severe) | 2 (gliosis) | 2 (mainly in perivascular globoid cells) |
| CW16 066 | 3 (severe) | 2 (gliosis) | 2 (mainly in perivascular globoid cells) |
| CW16 061 | 2 (moderate) | 2 (gliosis) | 2 (mainly in perivascular globoid cells) |
| CW16 065 | 1 (mild to moderate) | 2 (gliosis) | 1 (mainly in perivascular globoid cells) |
| CW18 064 | 1 (modest) | 2 (gliosis) | 1 (only few inflammatory cells) |
| CW16 062 | 0 (no evidence) | 1 (moderate gliosis) | 1 (only few inflammatory cells) |
| CW15 103 | 0 (no evidence) | 1 (mild gliosis) | 0 (no inflammatory cells) |
| Cerebrum * | Spinal Cord * | |
|---|---|---|
| hPTX3 mice. | 5.7 ± 2.8 | 1.5 ± 0.8 |
| Galctwi/twi mice | 514.4 ± 288.1 | 281.6 ± 38.1 |
| hPTX3/Galctwi/twi mice | 552.0 ± 90.9 | 393.2 ± 170.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coltrini, D.; Chandran, A.M.K.; Belleri, M.; Poliani, P.L.; Cominelli, M.; Pagani, F.; Capra, M.; Calza, S.; Prioni, S.; Mauri, L.; et al. β-Galactosylceramidase Deficiency Causes Upregulation of Long Pentraxin-3 in the Central Nervous System of Krabbe Patients and Twitcher Mice. Int. J. Mol. Sci. 2022, 23, 9436. https://doi.org/10.3390/ijms23169436
Coltrini D, Chandran AMK, Belleri M, Poliani PL, Cominelli M, Pagani F, Capra M, Calza S, Prioni S, Mauri L, et al. β-Galactosylceramidase Deficiency Causes Upregulation of Long Pentraxin-3 in the Central Nervous System of Krabbe Patients and Twitcher Mice. International Journal of Molecular Sciences. 2022; 23(16):9436. https://doi.org/10.3390/ijms23169436
Chicago/Turabian StyleColtrini, Daniela, Adwaid Manu Krishna Chandran, Mirella Belleri, Pietro L. Poliani, Manuela Cominelli, Francesca Pagani, Miriam Capra, Stefano Calza, Simona Prioni, Laura Mauri, and et al. 2022. "β-Galactosylceramidase Deficiency Causes Upregulation of Long Pentraxin-3 in the Central Nervous System of Krabbe Patients and Twitcher Mice" International Journal of Molecular Sciences 23, no. 16: 9436. https://doi.org/10.3390/ijms23169436
APA StyleColtrini, D., Chandran, A. M. K., Belleri, M., Poliani, P. L., Cominelli, M., Pagani, F., Capra, M., Calza, S., Prioni, S., Mauri, L., Prinetti, A., Kofler, J. K., Escolar, M. L., & Presta, M. (2022). β-Galactosylceramidase Deficiency Causes Upregulation of Long Pentraxin-3 in the Central Nervous System of Krabbe Patients and Twitcher Mice. International Journal of Molecular Sciences, 23(16), 9436. https://doi.org/10.3390/ijms23169436