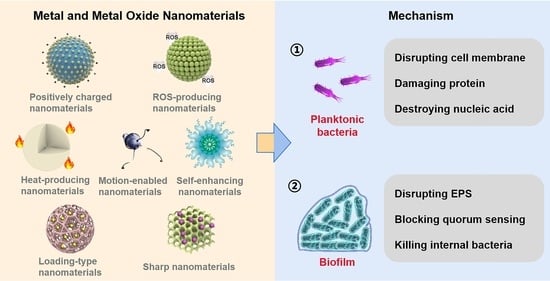
Metal and Metal Oxide Nanomaterials for Fighting Planktonic Bacteria and Biofilms: A Review Emphasizing on Mechanistic Aspects
Abstract
:1. Introduction
2. Metal and Metal Oxide Nanomaterials
2.1. Metal Nanomaterials
2.2. Metal Oxide Nanomaterials
2.3. Synthetic Method
3. Planktonic Bacteria
3.1. Cell Membrane

3.2. Protein
3.3. Nucleic Acid
4. Biofilm
4.1. Extracellular Polymeric Substance
4.2. Quorum Sensing
4.3. Bacteria
5. Conclusions and Future Perspectives
- Toxicity issues. The toxicity of metal and metal oxide nanomaterials should not be disregarded, despite the fact that they offer outstanding anti-infection properties, such as Ag nanomaterials. Hydrogels are crosslinked polymers that have outstanding biocompatibility and can hold a lot of water in solution without dissolving. It can be modified to provide a slow and controlled release of the load, such as temperature-sensitive hydrogels. Therefore, the encapsulation of metal and metal oxide nanomaterials in hydrogels to control their release behavior will avoid the toxic side effects of sudden release.
- Metabolic issues. A further issue is the metabolism of metal ions produced in vivo by the breakdown of metal nanomaterials. Fe, Cu, Zn, and Mo are essential trace elements for the human body. They show excellent antibacterial properties when formed as nanomaterials. For example, iron-based nanomaterials and copper-based nanomaterials present enzyme-like activities that can convert H2O2 into more toxic hydroxyl radicals for removing bacteria from the wound area. Thus, these nanomaterials should be given priority in the selection of anti-infective materials.
- Stability issues. Metal and metal oxide nanomaterials are usually unstable and prone to aggregation, especially in small sizes. However, it has been demonstrated that small-sized nanomaterials have a higher likelihood of passing through the membranes of bacterial cells and biofilms. As a result, the aggregation of metal and metal oxide nanomaterials will reduce their efficacy and even increase the toxic side effects. The nanocomposite created by combining metal and metal oxide nanostructures with other nanomaterials such as graphene would be a good choice. This approach not only ensures the dispersion of metal and metal oxide nanomaterials, but also enhances the anti-infective efficacy. In addition, performing ligand modification or wrapping organic layers such as PLGA is also a proper solution.
- Efficacy issues. The efficacy of metal and metal oxide nanomaterials that work with hyperthermia or ROS is limited by the distance of action. Fortunately, the distance can be reduced by the surface modification of metal and metal oxide material, such as ligand modification and increased surface roughness. This improvement will maximize the anti-infective properties of the nanomaterials while simultaneously maintaining biosafety.
- Mechanistic explanation. The currently available anti-infective mechanisms lack integrity, which are mostly at the cellular and protein levels. There is an urgent need to construct a complete and systematic anti-infective mechanism of metal and metal oxide nanomaterials based on the gene level, protein level, and cellular level. For example, researchers can study the expression of related genes and proteins from the signaling pathways related to the energy metabolism in the bacterium and explore the effect of materials on the energy production of the bacterium. In addition, researchers can study the redox homeostasis system of the bacterium and elaborate the mechanism of disruption of the antioxidant system of the bacterium by the material to lay the foundation for the design of the material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial resistance in bacteria: Mechanisms, evolution, and persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Yang, M.; Yan, D.; Yang, L.; Wan, X.; Xiao, J.; Yao, Y.; Luo, J. Surface-charge-switchable and size-transformable thermosensitive nanocomposites for chemo-photothermal eradication of bacterial biofilms in vitro and in vivo. ACS Appl. Mater. Interfaces 2022, 14, 8847–8864. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Hu, E.; Xie, R.; Yu, K.; Lu, F.; Bao, R.; Wang, C.; Lan, G.; Dai, F. Magnetically guided nanoworms for precise delivery to enhance in situ production of nitric oxide to combat focal bacterial infection in vivo. ACS Appl. Mater. Interfaces 2021, 13, 22225–22239. [Google Scholar] [CrossRef] [PubMed]
- Mahamuni-Badiger, P.P.; Patil, P.M.; Badiger, M.V.; Patel, P.R.; Thorat-Gadgil, B.S.; Pandit, A.; Bohara, R.A. Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles. Mater. Sci. Eng. C Mater. 2020, 108, 110319. [Google Scholar] [CrossRef] [PubMed]
- Ruhal, R.; Kataria, R. Biofilm patterns in gram-positive and gram-negative bacteria. Microbiol. Res. 2021, 251, 126829. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.J.; Barreto, R.T.; Barrois, B.M.; Gryson, L.G.; Meaume, S.; Monstrey, S.J. Update on the role of antiseptics in the management of chronic wounds with critical colonisation and/or biofilm. Int. Wound J. 2021, 18, 342–358. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Xie, S. Current progress and prospects of organic nanoparticles against bacterial biofilm. Adv. Colloid Interface Sci. 2021, 294, 102475. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Silva, N.B.S.; Marques, L.A.; Röder, D.D.B. Diagnosis of biofilm infections: Current methods used, challenges and perspectives for the future. J. Appl. Microbiol. 2021, 131, 2148–2160. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C.; Davidson, D.J.; Liddle, A.D. Recent strategies to combat infections from biofilm-forming bacteria on orthopaedic implants. Int. J. Mol. Sci. 2021, 22, 10243. [Google Scholar] [CrossRef]
- Johnson, C.T.; Wroe, J.A.; Agarwal, R.; Martin, K.E.; Guldberg, R.E.; Donlan, R.M.; Westblade, L.F.; Garcia, A.J. Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing. Proc. Natl. Acad. Sci. USA 2018, 115, E4960–E4969. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Preda, V.G.; Sandulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Mihai, M.M.; Holban, A.M.; Giurcaneanu, C.; Popa, L.G.; Buzea, M.; Filipov, M.; Lazar, V.; Chifiriuc, M.C.; Popa, M.I. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom. J. Morphol. Embryol. 2014, 55, 1401–1408. [Google Scholar]
- Alizadeh, N.; Salimi, A. Multienzymes activity of metals and metal oxide nanomaterials: Applications from biotechnology to medicine and environmental engineering. J. Nanobiotechnol. 2021, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qiao, Y.; Zhang, H.; Zhao, J.; Li, W.; Xie, T.; Zhong, D.; Wei, Q.; Hua, S.; Yu, Y.; et al. Gold-silver nanoshells promote wound healing from drug-resistant bacteria infection and enable monitoring via surface-enhanced Raman scattering imaging. Biomaterials 2020, 234, 119763. [Google Scholar] [CrossRef]
- Ali, S.G.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; AlYahya, S.; Jalal, M.; Khan, H.M.; Asiri, S.M.M.; Ahmad, W.; Mahdi, A.A.; et al. Biogenic gold nanoparticles as potent antibacterial and antibiofilm nano-antibiotics against pseudomonas aeruginosa. Antibiotics 2020, 9, 100. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, L.; Wang, Y.A.; Song, X.Y.; Li, K.Y.; Yan, X.F.; Yu, L.M.; He, Z.Y. Nanomaterial-based strategies in antimicrobial applications: Progress and perspectives. Nano Res. 2021, 14, 4417–4441. [Google Scholar] [CrossRef]
- Weldick, P.J.; Wang, A.H.; Halbus, A.F.; Paunov, V.N. Emerging nanotechnologies for targeting antimicrobial resistance. Nanoscale 2022, 14, 4018–4041. [Google Scholar] [CrossRef] [PubMed]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.N.; Shi, Y.R.; Song, H.; Yu, C.Z. Antibiotic-free antibacterial strategies enabled by nanomaterials: Progress and perspectives. Adv. Mater. 2020, 32, 1904106. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, X.Z.; He, C.; Huang, J.B.; Yin, S.Q.; Zhou, M.; Ma, L.; Zhao, W.F.; Qiu, L.; Cheng, C.; et al. Metal-organic framework/Ag-based hybrid nanoagents for rapid and synergistic bacterial eradication. ACS Appl. Mater. Interfaces 2020, 12, 13698–13708. [Google Scholar] [CrossRef]
- Ielo, I.; Rando, G.; Giacobello, F.; Sfameni, S.; Castellano, A.; Galletta, M.; Drommi, D.; Rosace, G.; Plutino, M.R. Synthesis, chemical-physical characterization, and biomedical applications of functional gold nanoparticles: A review. Molecules 2021, 26, 5823. [Google Scholar] [CrossRef]
- Czechowska, J.; Cichoń, E.; Belcarz, A.; Ślósarczyk, A.; Zima, A. Effect of gold nanoparticles and silicon on the bioactivity and antibacterial properties of hydroxyapatite/chitosan/tricalcium phosphate-based biomicroconcretes. Materials 2021, 14, 3854. [Google Scholar] [CrossRef]
- Li, J.J.; Cha, R.T.; Zhao, X.H.; Guo, H.B.; Luo, H.Z.; Wang, M.Z.; Zhou, F.S.; Jiang, X.Y. Gold Nanoparticles cure bacterial infection with benefit to intestinal microflora. ACS Nano 2019, 13, 5002–5014. [Google Scholar] [CrossRef]
- Xie, Y.Z.Y.; Zhang, Q.; Zheng, W.F.; Jiang, X.Y. Small molecule-capped gold nanoclusters for curing skin infections. ACS Appl. Mater. Interfaces 2021, 13, 35306–35314. [Google Scholar] [CrossRef]
- Zhang, X.C.; Zhang, Z.C.; Shu, Q.M.; Xu, C.; Zheng, Q.Q.; Guo, Z.; Wang, C.; Hao, Z.X.; Liu, X.; Wang, G.Q.; et al. Copper clusters: An effective antibacterial for eradicating multidrug-resistant bacterial infection in vitro and in vivo. Adv. Funct. Mater. 2021, 31, 2008720. [Google Scholar] [CrossRef]
- Nie, X.L.; Wu, S.L.; Liao, S.Q.; Chen, J.F.; Huang, F.L.; Li, W.; Wang, Q.Q.; Wei, Q.F. Light-driven self-disinfecting textiles functionalized by PCN-224 and Ag nanoparticles. J. Hazard. Mater. 2021, 416, 125786. [Google Scholar] [CrossRef]
- Wilke, C.M.; Wunderlich, B.; Gaillard, J.F.; Gray, K.A. Synergistic bacterial stress results from exposure to nano-Ag and nano-TiO2 mixtures under light in environmental media. Environ. Sci. Technol. 2018, 52, 3185–3194. [Google Scholar] [CrossRef]
- Yang, T.Y.; Hsieh, Y.J.; Lu, P.L.; Lin, L.; Wang, L.C.; Wang, H.Y.; Tsai, T.H.; Shih, C.J.; Tseng, S.P. In vitro and in vivo assessments of inspired Ag/80S bioactive nanocomposites against carbapenem-resistant Klebsiella pneumoniae. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 125, 112093. [Google Scholar] [CrossRef]
- Biao, L.H.; Tan, S.N.; Wang, Y.L.; Guo, X.M.; Fu, Y.J.; Xu, F.J.; Zu, Y.G.; Liu, Z.G. Synthesis, characterization and antibacterial study on the chitosan-functionalized Ag nanoparticles. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 76, 73–80. [Google Scholar] [CrossRef]
- Sarker, S.R.; Polash, S.A.; Boath, J.; Kandjani, A.E.; Poddar, A.; Dekiwadia, C.; Shukla, R.; Sabri, Y.; Bhargava, S.K. Functionalization of elongated tetrahexahedral Au nanoparticles and their antimicrobial activity assay. ACS Appl. Mater. Interfaces 2019, 11, 13450–13459. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Petralia, S.; Neri, G.; Corsaro, C.; Forte, L.; Squarzoni, S.; Guglielmino, S.; Traina, F.; Fazio, E.; et al. Antimicrobial effect and cytotoxic evaluation of Mg-doped hydroxyapatite functionalized with Au-nano rods. Molecules 2021, 26, 1099. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Akbari-Fakhrabadi, A.; Carrasco-Sanchez, V.; Amalraj, J. Hydroxypropyl methylcellulose-copper nanoparticle and its nanocomposite hydrogel films for antibacterial application. Carbohydr. Polym. 2021, 254, 117302. [Google Scholar] [CrossRef]
- Vergara-Llanos, D.; Koning, T.; Pavicic, M.F.; Bello-Toledo, H.; Diaz-Gomez, A.; Jaramillo, A.; Melendrez-Castro, M.; Ehrenfeld, P.; Sanchez-Sanhueza, G. Antibacterial and cytotoxic evaluation of copper and zinc oxide nanoparticles as a potential disinfectant material of connections in implant provisional abutments: An in-vitro study. Arch. Oral Biol. 2021, 122, 105031. [Google Scholar] [CrossRef]
- Prakash, V.; Kumari, A.; Kaur, H.; Kumar, M.; Gupta, S.; Bala, R. Green Synthesis, Characterization and antimicrobial activities of copper nanoparticles from the rhizomes extract of picrorhiza kurroa. Pharm. Nanotechnol. 2021, 9, 298–306. [Google Scholar] [CrossRef]
- Lin, Z.J.; Liu, L.Z.; Wang, W.; Jia, L.; Shen, Y.Q.; Zhang, X.M.; Ge, D.T.; Shi, W.; Sun, Y.A. The role and mechanism of polydopamine and cuttlefish ink melanin carrying copper ion nanoparticles in antibacterial properties and promoting wound healing. Biomater. Sci. 2021, 9, 5951–5964. [Google Scholar] [CrossRef]
- Fang, G.; Li, W.F.; Shen, X.M.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.F.; Chai, Z.F.; Chen, C.Y.; Ge, C.C.; Zhou, R.H. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat. Commun. 2018, 9, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Chu, Q.; Fang, C.; Cao, G.D.; Han, G.R.; Li, X. Cu-Ferrocene-Functionalized CaO2 Nanoparticles to Enable Tumor-Specific Synergistic Therapy with GSH Depletion and Calcium Overload. Adv. Sci. 2021, 8, 2100241. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Arooj, A.; Tahir, K.; Ibrahim, M.M.; Jevtovic, V.; Al-Abdulkarim, H.A.; Saleh, E.A.M.; Al-Shehri, H.S.; Amin, M.A.; Li, B.S. Facile fabrication of novel Ag2S-ZnO/GO nanocomposite with its enhanced photocatalytic and biological applications. J. Mol. Struct. 2022, 1251, 131991. [Google Scholar] [CrossRef]
- Peng, H.; Rossetto, D.; Mansy, S.S.; Jordan, M.C.; Roos, K.P.; Chen, I.A. Treatment of wound infections in a mouse model using Zn2+-releasing phage bound to gold nanorods. ACS Nano 2022, 16, 4756–4774. [Google Scholar] [CrossRef]
- Fontecha-Umana, F.; Rios-Castillo, A.G.; Ripolles-Avila, C.; Rodriguez-Jerez, J.J. Antimicrobial activity and prevention of bacterial biofilm formation of silver and zinc oxide nanoparticle-containing polyester surfaces at various concentrations for use. Foods 2020, 9, 442. [Google Scholar] [CrossRef]
- Saha, R.K.; Debanath, M.K.; Paul, B.; Medhi, S.; Saikia, E. Antibacterial and nonlinear dynamical analysis of flower and hexagon-shaped ZnO microstructures. Sci. Rep. 2020, 10, 2598. [Google Scholar] [CrossRef]
- Tong, T.Z.; Wilke, C.M.; Wu, J.S.; Binh, C.T.T.; Kelly, J.J.; Gaillard, J.F.; Gray, K.A. Combined toxicity of nano-ZnO and nano-TiO2: From Single- to Multinanomaterial Systems. Environ. Sci. Technol. 2015, 49, 8113–8123. [Google Scholar] [CrossRef]
- Hebeish, A.A.; Abdelhady, M.M.; Youssef, A.M. TiO2 nanowire and TiO2 nanowire doped Ag-PVP nanocomposite for antimicrobial and self-cleaning cotton textile. Carbohydr. Polym. 2013, 91, 549–559. [Google Scholar] [CrossRef]
- Ng, A.M.C.; Chan, C.M.N.; Guo, M.Y.; Leung, Y.H.; Djurisic, A.B.; Hu, X.; Chan, W.K.; Leung, F.C.C.; Tong, S.Y. Antibacterial and photocatalytic activity of TiO2 and ZnO nanomaterials in phosphate buffer and saline solution. Appl. Microbiol. Biotechnol. 2013, 97, 5565–5573. [Google Scholar] [CrossRef]
- Mori, Y.; Tagawa, T.; Fujita, M.; Kuno, T.; Suzuki, S.; Matsui, T.; Ishihara, M. Simple and environmentally friendly preparation and size control of silver nanoparticles using an inhomogeneous system with silver-containing glass powder. J. Nanopart. Res. 2011, 13, 2799–2806. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Khalaj, M. Green synthesis of copper nanoparticles using aqueous extract of the leaves of Euphorbia esula L and their catalytic activity for ligand-free Ullmann-coupling reaction and reduction of 4-nitrophenol. RSC Adv. 2014, 4, 47313–47318. [Google Scholar] [CrossRef]
- Din, M.; Arshad, F.; Hussain, Z.; Mukhtar, M. Green adeptness in the synthesis and stabilization of copper nanoparticles: Catalytic, antibacterial, cytotoxicity, and antioxidant activities. Nanoscale Res. Lett. 2017, 12, 638. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E-coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Wang, L.N.; Muhammed, M. Synthesis of zinc oxide nanoparticles with controlled morphology. J. Mater. Chem. 1999, 9, 2871–2878. [Google Scholar] [CrossRef]
- Harandi, F.N.; Khorasani, A.C.; Shojaosadati, S.A.; Hashemi-Najafabadi, S. Living Lactobacillus-ZnO nanoparticles hybrids as antimicrobial and antibiofilm coatings for wound dressing application. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 130, 112457. [Google Scholar] [CrossRef]
- Fan, J.N.; Cheng, Y.Q.; Sun, M.T. Functionalized gold nanoparticles: Synthesis, properties and biomedical applications. Chem. Rec. 2020, 20, 1474–1504. [Google Scholar] [CrossRef]
- van der Meer, S.B.; Hadrovic, I.; Meiners, A.; Loza, K.; Heggen, M.; Knauer, S.K.; Bayer, P.; Schrader, T.; Beuck, C.; Epple, M. New tools to probe the protein purface: Ultrasmall gold nanoparticles carry amino acid binders. J. Phys. Chem. B 2021, 125, 115–127. [Google Scholar] [CrossRef]
- Friedman, N.; Dagan, A.; Elia, J.; Merims, S.; Benny, O. Physical properties of gold nanoparticles affect skin penetration via hair follicles. Nanomed.-NBM 2021, 36, 102414. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.G.; Sun, J.B. Enhanced NO2 detection using hierarchical porous ZnO nanoflowers modified with graphene. Ceram. Int. 2016, 42, 9851–9857. [Google Scholar] [CrossRef]
- Mehta, D.; Saini, V.; Aggarwal, B.; Khan, A.; Bajaj, A. Unlocking the bacterial membrane as a therapeutic target for next-generation antimicrobial amphiphiles. Mol. Asp. Med. 2021, 81, 100999. [Google Scholar] [CrossRef]
- Liu, M.; Guo, L.K.; Fu, Y.X.; Huo, M.T.; Qi, Q.S.; Zhao, G. Bacterial protein acetylation and its role in cellular physiology and metabolic regulation. Biotechnol. Adv. 2021, 53, 107842. [Google Scholar] [CrossRef]
- Landick, R. Transcriptional pausing as a mediator of bacterial gene regulation. Annu. Rev. Microbiol. 2021, 75, 291–314. [Google Scholar] [CrossRef]
- Zhao, B.; Zheng, K.; Liu, C.G. Bio-dissolution process and mechanism of copper phosphate hybrid nanoflowers by Pseudomonas aeruginosa and its bacteria-toxicity in life cycle. J. Hazard. Mater. 2021, 419, 126494. [Google Scholar] [CrossRef]
- Guo, Y.D.; Xu, Y.Y.; Bao, Q.Q.; Shen, C.; Ni, D.L.; Hu, P.; Shi, J.L. Endogenous copper for nanocatalytic oxidative damage and self-protection pathway breakage of cancer. ACS Nano 2021, 15, 16286–16297. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Wang, X.; Liu, D. Release strategies of silver ions from materials for bacterial killing. ACS Appl. Bio Mater. 2021, 4, 3985–3999. [Google Scholar] [CrossRef]
- Haidari, H.; Bright, R.; Kopecki, Z.; Zilm, P.S.; Garg, S.; Cowin, A.J.; Vasilev, K.; Goswami, N. Polycationic silver nanoclusters comprising nanoreservoirs of Ag ions with high antimicrobial and antibiofilm activity. ACS Appl. Mater. Interfaces 2021, 14, 390–403. [Google Scholar] [CrossRef]
- Chandraker, K.; Nagwanshi, R.; Jadhav, S.K.; Ghosh, K.K.; Satnami, M.L. Antibacterial properties of amino acid functionalized silver nanoparticles decorated on graphene oxide sheets. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2017, 181, 47–54. [Google Scholar] [CrossRef]
- Nie, X.; Gao, F.; Wang, F.; Liu, C.; You, Y.Z. Charge-reversal silver clusters for targeted bacterial killing. J. Mater. Chem. B 2021, 9, 4006–4014. [Google Scholar] [CrossRef]
- Tu, Y.S.; Li, P.; Sun, J.J.; Jiang, J.; Dai, F.F.; Li, C.Z.; Wu, Y.Y.; Chen, L.; Shi, G.S.; Tan, Y.W.; et al. Remarkable antibacterial activity of reduced graphene oxide functionalized by copper ions. Adv. Funct. Mater. 2021, 31, 2008018. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Liao, Q.; Wu, L.; Luo, Y.X.; Zhang, W.; Guan, M.; Pan, H.B.; Tong, L.P.; Chu, P.K.; Wang, H.Y. ZnL2-BPs integrated bone scaffold under sequential photothermal mediation: A win-win strategy delivering antibacterial therapy and fostering osteogenesis thereafter. ACS Nano 2021, 15, 17854–17869. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, X.Y.; Liu, Z.X.; Xu, Y.H. Visible-light-driven photocatalysis-enhanced nanozyme of TiO2 Nanotubes@MoS2 nanoflowers for efficient wound healing infected with multidrug-resistant bacteria. Small 2021, 17, 2103348. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.S.; Xu, H.H.; Sun, B.; Du, S.L.; Cui, S.; Zhang, L.; Ding, N.; Yang, D.Z. pH-responsive oxygen and hydrogen peroxide self-supplying nanosystem for photodynamic and chemodynamic Ttherapy of wound infection. ACS Appl. Mater. Interfaces 2021, 13, 59720–59730. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, S.; Liu, Y.; Meng, J.S.; Wang, M.X.; Sun, Y.J.; Xia, L.B.; He, Z.Z.; Hu, W.X.; Ren, L.; et al. Antibacterial cascade catalytic glutathione-depleting MOF nanoreactors. ACS Appl. Mater. Interfaces 2022, 14, 11104–11115. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Liu, H.L.; Liao, K.D.; Hu, Q.Q.; Guo, R.; Deng, K.X. Functionalized GO nanovehicles with nitric oxide release and photothermal activity-based hydrogels for bacteria-infected wound healing. ACS Appl. Mater. Interfaces 2020, 12, 28952–28964. [Google Scholar] [CrossRef]
- Du, T.; Huang, B.; Cao, J.; Li, C.; Jiao, J.; Xiao, Z.; Wei, L.; Ma, J.; Du, X.; Wang, S. Ni Nanocrystals supported on graphene oxide: Antibacterial agents for synergistic treatment of bacterial infections. ACS Omega 2022, 7, 18339–18349. [Google Scholar] [CrossRef] [PubMed]
- Hasija, V.; Kumar, A.; Sudhaik, A.; Raizada, P.; Singh, P.; Le, Q.V.; Le, T.T.; Nguyen, V.H. Step-scheme heterojunction photocatalysts for solar energy, water splitting, CO2 conversion, and bacterial inactivation: A review. Environ. Chem. Lett. 2021, 19, 2941–2966. [Google Scholar] [CrossRef]
- Sun, G.H.; Jia, S.S.; Zhang, X.Y.; Kang, Z.W.; Cui, M.L.; Wang, B.Q.; Wang, B.; Yang, D.P. Anchoring core-shell Cu@Cu2O nanoparticles to two-dimensional carbon nanosheets for bacterial disinfection. ACS Appl. Nano Mater. 2021, 4, 9831–9841. [Google Scholar] [CrossRef]
- Short, F.L.; Lee, V.; Mamun, R.; Malmberg, R.; Li, L.P.; Espinosa, M.I.; Venkatesan, K.; Paulsen, I.T.; Macquarie Univ Undergrad, B. Benzalkonium chloride antagonises aminoglycoside antibiotics and promotes evolution of resistance. Ebiomedicine 2021, 73, 103653. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, W.F.; Li, S.X.; Zhong, L.N.; Jiang, X.Y. Aminophenol-Decorated gold nanoparticles for curing bacterial infections. Nano Lett. 2022, 22, 3576–3582. [Google Scholar] [CrossRef]
- Lepelley, A.; Della Mina, E.; Van Nieuwenhove, E.; Waumans, L.; Fraitag, S.; Rice, G.I.; Dhir, A.; Fremond, M.L.; Rodero, M.P.; Seabra, L.; et al. Enhanced cGAS-STING-dependent interferon signaling associated with mutations in ATAD3A. J. Exp. Med. 2021, 218, e20201560. [Google Scholar] [CrossRef]
- Guijarro, J.A.; Cascales, D.; Garcia-Torrico, A.I.; Garcia-Dominguez, M.; Mendez, J. Temperature-dependent expression of virulence genes in fish-pathogenic bacteria. Front. Microbiol. 2015, 6, 700. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Laser Assisted Synthesis, Imaging and cancer therapy of magnetic nanoparticles. Mater. Focus 2016, 5, 305–323. [Google Scholar] [CrossRef]
- Asensio, J.M.; Marbaix, J.; Mille, N.; Lacroix, L.M.; Soulantica, K.; Fazzini, P.F.; Carrey, J.; Chaudret, B. To heat or not to heat: A study of the performances of iron carbide nanoparticles in magnetic heating. Nanoscale 2019, 11, 5402–5411. [Google Scholar] [CrossRef]
- Sun, C.X.; Wang, W.Q.; Sun, X.L.; Chu, W.H.; Yang, J.; Dai, J.J.; Ju, Y.M. An intrinsically thermogenic nanozyme for synergistic antibacterial therapy. Biomater. Sci. 2021, 9, 8323–8334. [Google Scholar] [CrossRef]
- Husak, V.V.; Mosiichuk, N.M.; Kubrak, O.I.; Matviishyn, T.M.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Acute exposure to copper induces variable intensity of oxidative stress in goldfish tissues. Fish Physiol. Biochem. 2018, 44, 841–852. [Google Scholar] [CrossRef]
- Murakami, K.; Tsubouchi, R.; Fukayama, M.; Yoshino, M. Copper-dependent inhibition and oxidative inactivation with affinity cleavage of yeast glutathione reductase. Biometals 2014, 27, 551–558. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Setyawati, M.I.; Leong, D.T.; Xie, J.P. Antimicrobial gold nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef]
- Emmanuel, R.; Palanisamy, S.; Chen, S.M.; Chelladurai, K.; Padmavathy, S.; Saravanan, M.; Prakash, P.; Ali, M.A.; Al-Hemaid, F.M.A. Antimicrobial efficacy of green synthesized drug blended silver nanoparticles against dental caries and periodontal disease causing microorganisms. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 56, 374–379. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Liao, B.Y.; Ye, X.C.; Chen, X.; Zhou, Y.J.; Cheng, L.; Zhou, X.D.; Ren, B.A. The two-component signal transduction system and its regulation in Candida albicans. Virulence 2021, 12, 1884–1899. [Google Scholar] [CrossRef]
- Sanchez-Garcia, F.J.; Perez-Hernandez, C.A.; Rodriguez-Murillo, M.; Moreno-Altamirano, M.M.B. The role of tricarboxylic acid cycle metabolites in viral infections. Front. Cell. Infect. Microbiol. 2021, 11, 725043. [Google Scholar] [CrossRef]
- Tu, C.X.; Lu, H.D.; Zhou, T.; Zhang, W.Y.; Deng, L.W.; Cao, W.B.; Yang, Z.J.; Wang, Z.L.; Wu, X.Y.; Ding, J.; et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials 2022, 286, 121597. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Su, Y.B.; Li, H.; Han, Y.; Guo, C.; Tian, Y.M.; Peng, X.X. Exogenous alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metab. 2015, 21, 249–262. [Google Scholar] [CrossRef]
- Li, X.; Ahmad, K.Z.; He, J.; Li, H.X.; Wang, X.; Feng, Z.J.; Wang, X.S.; Shen, G.X.; Ding, X.T. Silver nanoflowers coupled with low dose antibiotics enable the highly effective eradication of drug-resistant bacteria. J. Mater. Chem. B 2021, 9, 9839–9851. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Rizzello, L.; Pompa, P.P. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 2014, 43, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- Arnaouteli, S.; Bamford, N.C.; Stanley-Wall, N.R.; Kovacs, A.T. Bacillus subtilis biofilm formation and social interactions. Nat. Rev. Microbiol. 2021, 19, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef]
- Yuan, Z.; Lin, C.C.; Dai, L.L.; He, Y.; Hu, J.W.; Xu, K.; Tao, B.L.; Liu, P.; Cai, K.Y. Near-infraredlight-activatable dual-action nanoparticle combats the established biofilms of methicillin-resistant Staphylococcus aureus and its accompanying inflammation. Small 2021, 17, 2007522. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Li, B.Y.; Geng, M.D.; Wang, Y.; Zhao, J.G.; Jin, B.D.; Li, Y.F. Response of extracellular polymeric substances and microbial community structures on resistance genes expression in wastewater treatment containing copper oxide nanoparticles and humic acid. Bioresour. Technol. 2021, 340, 125741. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Chan, K.F.; Schweizer, K.; Du, X.Z.; Jin, D.D.; Yu, S.C.H.; Nelson, B.J.; Zhang, L. Ultrasound doppler-guided real-time navigation of a magnetic microswarm for active endovascular delivery. Sci. Adv. 2021, 7, eabe5914. [Google Scholar] [CrossRef]
- Naha, P.C.; Liu, Y.; Hwang, G.; Huang, Y.; Gubara, S.; Jonnakuti, V.; Simon-Soro, A.; Kim, D.; Gao, L.Z.; Koo, H.; et al. Dextran-coated iron oxide nanoparticles as biomimetic catalysts for localized and pH-activated biofilm disruption. ACS Nano 2019, 13, 4960–4971. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, L.; Yuan, K.; Ji, F.T.; Gao, J.H.; Zhang, Z.F.; Du, X.Z.; Tian, Y.; Wang, Q.Q.; Zhang, L. Magnetic microswarm composed of porous nanocatalysts for targeted elimination of biofilm occlusion. ACS Nano 2021, 15, 5056–5067. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Xia, Y.; Lan, Y.C.; Mokeem, L.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Magnetic-responsive photosensitizer nanoplatform for optimized inactivation of dental caries-related biofilms: Technology development and proof of principle. ACS Nano 2021, 15, 19888–19904. [Google Scholar] [CrossRef]
- Guo, G.Y.; Zhang, H.L.; Shen, H.; Zhu, C.Z.; He, R.K.; Tang, J.; Wang, Y.; Jiang, X.W.; Wang, J.X.; Bu, W.B.; et al. Space-selective chemodynamic therapy of CuFe5O8 nanocubes for implant-related infections. ACS Nano 2020, 14, 13391–13405. [Google Scholar] [CrossRef]
- Shi, T.L.; Hou, X.; Guo, S.Q.; Zhang, L.; Wei, C.H.; Peng, T.; Hu, X.G. Nanohole-boosted electron transport between nanomaterials and bacteria as a concept for nano-bio interactions. Nat. Commun. 2021, 12, 493. [Google Scholar] [CrossRef]
- Xia, W.Y.; Li, N.Y.; Shan, H.J.; Lin, Y.W.; Yin, F.L.; Yu, X.W.; Zhou, Z.B. Gallium porphyrin and gallium nitrate reduce the high vancomycin tolerance of MRSA biofilms by promoting extracellular DNA-dependent biofilm dispersion. ACS Infect. Dis. 2021, 7, 2565–2582. [Google Scholar] [CrossRef]
- Czaran, T.; Hoekstra, R.F. A spatial model of the evolution of quorum sensing regulating bacteriocin production. Behav. Ecol. 2007, 18, 866–873. [Google Scholar] [CrossRef]
- Shah, S.; Gaikwad, S.; Nagar, S.; Kulshrestha, S.; Vaidya, V.; Nawani, N.; Pawar, S. Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogen Pseudomonas aeruginosa. Biofouling 2019, 35, 34–49. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Alajmi, M.F.; Khan, M.A.; Pervez, S.A.; Ahmed, F.; Amir, S.; Husain, F.M.; Khan, M.S.; Shaik, G.M.; Hassan, I.; et al. Biosynthesized silver nanoparticle from pandanus odorifer leaf extract exhibits anti-metastasis and anti-biofilm potentials. Front. Microbiol. 2019, 10, 8. [Google Scholar] [CrossRef]
- Samanta, S.; Singh, B.R.; Adholeya, A. Intracellular Synthesis of Gold Nanoparticles using an ectomycorrhizal strain EM-1083 of laccaria fraterna and its nanoanti-quorum sensing potential against pseudomonas aeruginosa. Indian J. Microbiol. 2017, 57, 448–460. [Google Scholar] [CrossRef]
- Ghaffarlou, M.; İlk, S.; Hammamchi, H.; Kıraç, F.; Okan, M.; Güven, O.; Barsbay, M. Green and facile synthesis of pullulan-stabilized silver and gold nanoparticles for the inhibition of quorum sensing. ACS Appl. Bio Mater. 2022, 5, 517–527. [Google Scholar] [CrossRef]
- Prateeksha, P.; Bajpai, R.; Rao, C.V.; Upreti, D.K.; Barik, S.K.; Singh, B.N. Chrysophanol-functionalized silver nanoparticles for anti-adhesive and anti-biofouling coatings to prevent urinary catheter-associated infections. ACS Appl. Nano Mater. 2021, 4, 1512–1528. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, S.; Karmacharya, M.; Dubbu, S.; Kwon, T.; Singh, V.; Chae, K.H.; Kumar, A.; Cho, Y.K.; Lee, I.S. Surface-textured mixed-metal-oxide nanocrystals as efficient catalysts for ROS production and biofilm eradication. Nano Lett. 2021, 21, 279–287. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Kim, D.; Ren, Z.; Oh, M.J.; Cormode, D.P.; Hara, A.T.; Zero, D.T.; Koo, H. Ferumoxytol nanoparticles target biofilms causing tooth decay in the human mouth. Nano Lett. 2021, 21, 9442–9449. [Google Scholar] [CrossRef]
- Wang, J.X.; Wang, L.T.; Pan, J.; Zhao, J.H.; Tang, J.; Jiang, D.J.; Hu, P.; Jia, W.T.; Shi, J.L. Magneto-based synergetic therapy for implant-associated infections via biofilm disruption and innate immunity regulation. Adv. Sci. 2021, 8, 2004010. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.Q.; Hua, Y.S.; Zhang, Y.T.; Lv, M.Z.; Wang, H.; Pi, Y.; Xie, J.N.; Wang, C.Y.; Yong, Y. A biofilm microenvironment-activated single-atom iron nanozyme with NIR-controllable nanocatalytic activities for synergetic bacteria-infected wound therapy. Adv. Healthc. Mater. 2021, 10, 2101374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Y.; Wang, Q.; Jiang, J.; Gao, L.Z. Nanozybiotics: Nanozyme-based antibacterials against bacterial resistance. Antibiotics 2022, 11, 390. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Chen, Z.; Zeng, S.; Wang, C.F.; Li, W.L.; Wang, M.; Wang, X.M.; Zhou, X.; Zhao, X.Q.; Ren, L. Optimization of nanostructured copper sulfide to achieve enhanced enzyme-mimic activities for improving anti-infection performance. ACS Appl. Mater. Interfaces 2021, 13, 53659–53670. [Google Scholar] [CrossRef]










| Nanomaterials | Synthetic Method | Mode of Action | Bacterial Species | Ref. |
|---|---|---|---|---|
| Ag NPs | Chemical reduction | Ag+ | E. coli and S. aureus | [33] |
| Nano-Ag | Photoreduction | Ag+ and ROS | E. coli | [34] |
| Ag NPs | Chemical reduction | ROS | Carbapenem-resistant K. pneumoniae | [35] |
| Ag NPs | Hydrothermal reaction | Ag+ | E. coli, S. aureus, and C. albicans | [36] |
| Au NPs | Seed-mediated growth | ROS | B. subtilis and E. coli | [37] |
| Au NPs | Chemical reduction | No information | E. coli | [28] |
| Au NCs | Chemical reduction | ROS | E. coli, S. aureus, MDR E. coli, and MDR S. aureus | [29] |
| Au NRs | Chemical reduction | ROS | E. coli and S. aureus | [38] |
| Cu NPs | Solution casting method | Free radicals | E. coli and S. aureus | [39] |
| Cu NPs | Atmosphere arc discharge method | ROS | S. sanguinis, P. gingivalis, and S. mutans | [40] |
| Cu NPs | Biosynthetic method | ROS | E. coli and S. aureus | [41] |
| Nanomaterials | Synthetic Method | Mode of Action | Bacterial Species | Ref. |
|---|---|---|---|---|
| ZnO NPs | Atmosphere arc discharge method | ROS | S. sanguinis, P. gingivalis, and S. mutans | [40] |
| ZnO NPs | Biosynthetic method | ROS and Zn+ | E. coli and S. aureus P. aeruginosa and C. albicans | [47] |
| Flower-shaped ZnO | Wet chemical method | ROS | E. coli | [48] |
| Nano-TiO2 | No information | ROS | E. coli and A. hydrophila | [49] |
| TiO2 nanowire | Hydrothermal reaction | ROS | Gram-positive bacteria and Gram-negative bacteria | [50] |
| TiO2 NPs | Purchased from Nanostructured and Amorphous Materials Inc. and MK Impex Corp., Division MK Nano | ROS | E. coli | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Wang, X.; Dai, J.; Ju, Y. Metal and Metal Oxide Nanomaterials for Fighting Planktonic Bacteria and Biofilms: A Review Emphasizing on Mechanistic Aspects. Int. J. Mol. Sci. 2022, 23, 11348. https://doi.org/10.3390/ijms231911348
Sun C, Wang X, Dai J, Ju Y. Metal and Metal Oxide Nanomaterials for Fighting Planktonic Bacteria and Biofilms: A Review Emphasizing on Mechanistic Aspects. International Journal of Molecular Sciences. 2022; 23(19):11348. https://doi.org/10.3390/ijms231911348
Chicago/Turabian StyleSun, Caixia, Xiaobai Wang, Jianjun Dai, and Yanmin Ju. 2022. "Metal and Metal Oxide Nanomaterials for Fighting Planktonic Bacteria and Biofilms: A Review Emphasizing on Mechanistic Aspects" International Journal of Molecular Sciences 23, no. 19: 11348. https://doi.org/10.3390/ijms231911348
APA StyleSun, C., Wang, X., Dai, J., & Ju, Y. (2022). Metal and Metal Oxide Nanomaterials for Fighting Planktonic Bacteria and Biofilms: A Review Emphasizing on Mechanistic Aspects. International Journal of Molecular Sciences, 23(19), 11348. https://doi.org/10.3390/ijms231911348