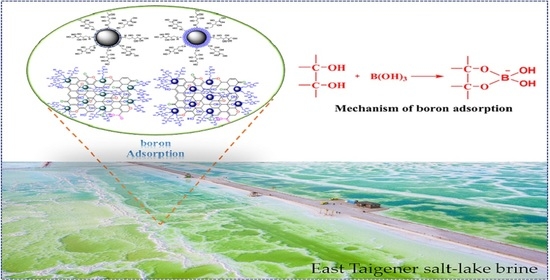
Magnetic Separation of Oxoacid of Boron from Salt-Lake Brine by Synergistically Enhanced Boron Adsorbents of Glucose-Functionalized SiO2 and Graphene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Adsorbents
2.2. Adsorption-Desorption Performance of the Adsorbents
3. Materials and Methods
3.1. Materials, Physicochemical Measurements, and Method of Determination of Boron
3.2. Synthesis of the Fe3O4@SiO2-Glu, Fe3O4@SiO2@mSiO2-Glu, Go-Fe3O4@SiO2-Glu, and Go-Fe3O4@SiO2@mSiO2-Glu
3.2.1. Preparation of Fe3O4
3.2.2. Preparation of Fe3O4@SiO2
3.2.3. Preparation of Fe3O4@SiO2@mSiO2
3.2.4. Preparation of Fe3O4@SiO2-NH2 and Fe3O4@SiO2@mSiO2-NH2
3.2.5. Preparation of Fe3O4@SiO2-Glu and Fe3O4@SiO2@ mSiO2-Glu
3.2.6. Preparation of Graphene
3.2.7. Preparation of Fe3O4-Graphene
3.2.8. Preparation of Go-Fe3O4@SiO2 and Go-Fe3O4@SiO2@mSiO2
3.2.9. Preparation of Go-Fe3O4@SiO2-NH2 and Go-Fe3O4@SiO2@mSiO2-NH2
3.2.10. Preparation of Go-Fe3O4@SiO2-Glu and Go-Fe3O4@SiO2@mSiO2-Glu
3.3. Boron Adsorption-Desorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilal, N.; Kim, G.J.; Somerfield, C. Boron Removal from Saline Water: A Comprehensive Review. Desalination 2011, 273, 23. [Google Scholar] [CrossRef]
- Luo, Q.L.; Cheng, Z.F.; He, L.L.; Wang, X.Y.; Li, K.H.; Huang, X.L. Glucose and glycidol grafted polyacrylonitrile particles by hydrothermal synthesis for enriched boron from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125976. [Google Scholar] [CrossRef]
- Nasef, M.M.; Nallappan, M.; Ujang, Z. Polymer-based chelating adsorbents for the selective removal of boron from water and wastewater: A review. React. Funct. Polym. 2014, 85, 54–68. [Google Scholar] [CrossRef]
- Yılmaz, A.E.; Boncukcuoglu, R.; Yılmaz, M.T.; Kocakerim, M.M. Adsorption of boron from boron-containing wastewaters by ion exchange in a continuous reactor. J. Hazard. Mater. 2005, 117, 221–226. [Google Scholar] [CrossRef]
- Schott, J.; Kretzschmar, J.; Acker, M.; Eidner, S.; Kumke, M.U.; Drobot, B.; Barkleit, A.; Taut, S.; Brendler, V.; Stumpf, T. Formation of a Eu(III) borate solid species from a weak Eu(III) borate complex in aqueous solution. Dalton Trans. 2014, 43, 11516–11528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.W.; Chen, S.F.; Bao, Z.B.; Xing, H.B.; Zhang, Z.G.; Su, B.G.; Yang, Q.W.; Yang, Y.W.; Ren, Q.L. A spherical N-methyl-D-glucamine-based hybrid adsorbent for highly efficient adsorption of boric acid from water. Sep. Purif. Technol. 2017, 172, 43–50. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, Y.; Song, J.; Xing, L.; Kong, D.; Li, X.-M.; He, T. Extraction of boron from salt lake brine using 2-ethylhexanol. Hydrometallurgy 2016, 160, 129–136. [Google Scholar] [CrossRef]
- Meng, J.Q.; Cao, J.J.; Xu, R.S.; Wang, Z.; Sun, R.B. Hyperbranched grafting enabling simultaneous enhancement of the boric acid uptake and the adsorption rate of a complexing membrane. J. Mater. Chem. A 2016, 4, 11656–11665. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Lyu, J.F.; Wang, Y.M.; Chen, T.; Tian, Y.; Bai, P.; Guo, X.H. Synthesis, Characterization, Adsorption, and Isotopic Separation Studies of Pyrocatechol-Modified MCM-41 for Efficient Boron Removal. Ind. Eng. Chem. Res. 2019, 58, 3282–3292. [Google Scholar] [CrossRef]
- Pan, Y.; Du, J.; Chen, J.; Lian, C.; Lin, S.; Yu, J. Interlayer intercalation of Li/Al-LDHs responsible for high-efficiency boron extraction. Desalination 2022, 539, 115966. [Google Scholar] [CrossRef]
- Bai, C.; Guo, M.; Liu, Z.; Wu, Z.J.; Li, Q. A novel method for removal of boron from aqueous solution using sodium dodecyl benzene sulfonate and D-mannitol as the collector. Desalination 2018, 431, 47–55. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Al-Ghouti, M.A.; Khan, M.; Kasak, P.; Krupa, I. Modified os sepiae of Sepiella inermis as a low cost, sustainable, bio-based adsorbent for the effective remediation of boron from aqueous solution. Environ. Sci. Pollut. Res. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; He, L.; Wang, X.; Huang, H.; Wang, X.; Sang, S.; Huang, X. Cyclodextrin derivatives used for the separation of boron and the removal of organic pollutants. Sci. Total Environ. 2020, 749, 141487. [Google Scholar] [CrossRef]
- Kamcev, J.; Taylor, M.K.; Shin, D.M.; Jarenwattananon, N.N.; Colwell, K.A.; Long, J.R. Functionalized Porous Aromatic Frameworks as High-Performance Adsorbents for the Rapid Removal of Boric Acid from Water. Adv. Mater. 2019, 31, e1808027. [Google Scholar] [CrossRef]
- Neo, J.G.; Japip, S.; Luo, L.; Chung, T.-S.; Weber, M.; Maletzko, C. Hydroxyl-terminated poly(ethyleneimine) polymer enhanced ultrafiltration for boron removal. Sep. Purif. Technol. 2019, 222, 214–220. [Google Scholar] [CrossRef]
- Lyu, J.F.; Zeng, Z.L.Z.; Zhang, N.; Liu, H.X.; Bai, P.; Guo, X.H. Pyrocatechol-modified resins for boron recovery from water: Synthesis, adsorption and isotopic separation studies. React. Funct. Polym 2017, 112, 1–8. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.Q.; Hu, H.P.; Wu, Z.P.; Chen, Q.Y. Synthesis, characterization and application of a novel silica based adsorbent for boron removal. Desalination 2012, 294, 1–7. [Google Scholar] [CrossRef]
- Chen, F.; Guo, L.; Zhang, X.; Leong, Z.Y.; Yang, S.; Yang, H.Y. Nitrogen-doped graphene oxide for effectively removing boron ions from seawater. Nanoscale 2017, 9, 326–333. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Y.; Lowe, S.E.; Jiang, L.; Ling, H.Y.; Shi, G.; Liu, P.; Zhang, S.; Zhong, Y.L.; Zhao, H. Room temperature production of graphene oxide with thermally labile oxygen functional groups for improved lithium ion battery fabrication and performance. J. Mater. Chem. A 2019, 7, 9646–9655. [Google Scholar] [CrossRef]
- Chen, H.; Du, W.; Liu, J.; Qu, L.; Li, C. Efficient room-temperature production of high-quality graphene by introducing removable oxygen functional groups to the precursor. Chem. Sci. 2019, 10, 1244–1253. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.-L.; Wang, L.; Chen, C.-L.; Xu, A.-W.; Wang, X.-K. Water-dispersible magnetite-graphene-LDH composites for efficient arsenate removal. J. Mater. Chem. 2011, 21, 17353–17359. [Google Scholar] [CrossRef]
- Yin, D.; Liu, H.; Zhang, B.; Geng, W. Magnetic boron specific chelating microsphere by dispersion polymerisation for boron adsorption. Mater. Technol. 2016, 31, 352–357. [Google Scholar] [CrossRef]
- Tan, X.; Lu, L.; Wang, L.; Zhang, J. Facile Synthesis of Bimodal Mesoporous Fe3O4@SiO2 Composite for Efficient Removal of Methylene Blue. Eur. J. Inorg. Chem. 2015, 2015, 2928–2933. [Google Scholar] [CrossRef]
- Luo, Q.L.; Zeng, M.T.; Wang, X.Y.; Huang, H.; Wang, X.F.; Liu, N.; Huang, X.L. Glycidol-functionalized macroporous polymer for boron removal from aqueous solution. React. Funct. Polym. 2020, 150, 104543. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.; Li, L.; Huang, X.; Cheng, Z.; Wang, X.; He, L. Hydrothermal synthesis of hydroxyl terminated polymer boron adsorbents. J. Solid State Chem. 2021, 296, 121977. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Saberi, V.; Kalhor, S.; Ayati, S.E. A novel highly dispersive magnetic nanocatalyst in water: glucose as an efficient and green ligand for the immobilization of copper(ii) for the cycloaddition of alkynes to azides. RSC Adv. 2016, 6, 80234–80243. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, N.; Chi, Y.; Geng, W.; Yan, W.; Zhao, Y.; Li, X.; Dong, B. Effect of large pore size of multifunctional mesoporous microsphere on removal of heavy metal ions. J. Hazard. Mater. 2013, 254–255, 157–165. [Google Scholar] [CrossRef]
- Nodeh, H.R.; Kamboh, M.A.; Wan Ibrahim, W.A.; Jume, B.H.; Sereshti, H.; Sanagi, M.M. Equilibrium, kinetic and thermodynamic study of pesticides removal from water using novel glucamine-calix[4]arene functionalized magnetic graphene oxide. Environ. Sci. Processes Impacts 2019, 21, 714–726. [Google Scholar] [CrossRef]
- Guo, L.; Li, D.; Lennholm, H.; Zhai, H.; Ek, M. Structural and functional modification of cellulose nanofibrils using graft copolymerization with glycidyl methacrylate by Fe2+–thiourea dioxide–H2O2 redox system. Cellulose 2019, 26, 4853–4864. [Google Scholar] [CrossRef]
- Wei, Y.T.; Zheng, Y.M.; Chen, J.P. Design and fabrication of an innovative and environmental friendly adsorbent for boron removal. Water Res. 2011, 45, 2297–2305. [Google Scholar] [CrossRef]
- Schaeffer, R. Boron, Metallo-Boron Compounds and Boranes. J. Am. Chem. Soc. 1965, 87, 3535–3536. [Google Scholar] [CrossRef]
- Christ, C.L.; Truesdell, A.H.; Erd, R.C. Borate mineral assemblages in the system Na2O-CaO-MgO-B2O3-H2O. Geochim. Cosmochim. Acta 1967, 31, 313–337. [Google Scholar] [CrossRef]
- Darwish, N.B.; Kochkodan, V.; Hilal, N. Boron removal from water with fractionized Amberlite IRA743 resin. Desalination 2015, 370, 1–6. [Google Scholar] [CrossRef]
- Kaftan, O.; Acikel, M.; Eroglu, A.E.; Shahwan, T.; Artok, L.; Ni, C.Y. Synthesis, characterization and application of a novel sorbent, glucamine-modified MCM-41, for the removal/preconcentration of boron from waters. Anal. Chim. Acta 2005, 547, 31–41. [Google Scholar] [CrossRef]
- Liu, H.N.; Ye, X.S.; Li, Q.; Kim, T.; Qing, B.J.; Guo, M.; Ge, F.; Wu, Z.J.; Lee, K. Boron adsorption using a new boron-selective hybrid gel and the commercial resin D564. Colloids Surf. A Physicochem. Eng. Asp. 2009, 341, 118–126. [Google Scholar] [CrossRef]
- Sanfeliu, C.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Amorós, P.; Azaïs, T.; Marcos, M.D. 11B-MAS NMR approach to the boron adsorption mechanism on a glucose-functionalised mesoporous silica matrix. Microporous Mesoporous Mater. 2018, 266, 232–241. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, L.; Dong, R.; Cheng, Z.; Dong, M.; Wu, Z.; Li, J.; Huang, X. A tris(hydroxymethyl)methyl aminomethane-functionalized SBA-15, SBA-16 and MCM-41 for recovery of boron from salt lake brine. Desalination Water Treat. 2022, 259, 116–126. [Google Scholar] [CrossRef]
- Sungur, Ş.; Okur, R. Using Azomethine-H Method Determination of Boron Contents of Various Foods Consumed in Hatay Region in Turkey. Food Chem. 2009, 115, 711. [Google Scholar] [CrossRef]
- Luo, Q.; Dong, M.; Li, Q.; Wu, Z.; Liu, Z.; Li, J. Improve the durability of lithium adsorbent Li/Al-LDHs by Fe3+ substitution and nanocomposite of FeOOH. Miner. Eng. 2022, 185, 107717. [Google Scholar] [CrossRef]







| Boron Adsorbents | BET Specific Surface Area, m2/g | Pore Volume, m3/g | Average Pore Size, nm |
|---|---|---|---|
| Fe3O4@SiO2@mSiO2-Glu | 29.28 | 0.017 | 1.696 |
| Go-Fe3O4@SiO2@mSiO2-Glu | 15.52 | 0.039 | 3.826 |
| No. | Adsorbent | Ligand | Q (mg/g) | T (min) | Reference |
|---|---|---|---|---|---|
| 1 | Amberlit IRA743 | NMDG | 5.41 | 60 | [33] |
| 2 | CL-MCM-41 | Pyrocatechol | 19.45 | 30 | [9] |
| 3 | MCM-41-NMDG | NMDG | 8.648 | 30 | [34] |
| 4 | D564-MG | NMDG | 12.43 | 100 | [35] |
| 5 | UVM-7-Glu | Glucose | 19.99 | - | [36] |
| 6 | Si-MG | NMDG | 16.65 | 120 | [34] |
| 7 | SBA-15-Tris | Tris | 15.28 | 80 | [37] |
| 8 | N-GO | -OH (N-doping) | 58.70 | 900 | [18] |
| 9 | Fe3O4@SiO2-Glu | Glucose | 16.68 | 60 | This work |
| Fe3O4@SiO2@mSiO2-Glu | Glucose | 20.99 | 60 | ||
| Go-Fe3O4@SiO2-Glu | Glucose | 18.91 | 60 | ||
| Go-Fe3O4@SiO2@mSiO2-Glu | Glucose | 23.90 | 60 |
| Adsorbents | Langmuir Isotherm Model | Freundlich Isotherm Model | ||||
|---|---|---|---|---|---|---|
| Qm/(mg·g−1) | KL/(L·mg−1) | R2 | KF | n | R2 | |
| Fe3O4@SiO2-Glu | 27.03 | 0.0022 | 0.999 | 0.5143 | 1.6340 | 0.981 |
| Fe3O4@SiO2@mSiO2-Glu | 32.38 | 0.0025 | 0.998 | 0.4435 | 1.6892 | 0.973 |
| Go-Fe3O4@SiO2-Glu | 28.90 | 0.0026 | 0.999 | 0.4231 | 1.7133 | 0.971 |
| Go-Fe3O4@SiO2@mSiO2-Glu | 36.67 | 0.0026 | 0.999 | 0.5143 | 1.6974 | 0.972 |
| Adsorbents | First-Order Model Fitting | Second-Order Model Fitting | ||||
|---|---|---|---|---|---|---|
| Qe,cal/mg/g | k1 | R2 | Qe,cal/mg/g | k2 | R2 | |
| Fe3O4@SiO2-Glu | 14.87 | 0.0557 | 0.957 | 16.14 | 0.0894 | 0.988 |
| Go-Fe3O4@SiO2-Glu | 16.79 | 0.0655 | 0.981 | 18.30 | 0.0992 | 0.988 |
| Fe3O4@SiO2@mSiO2-Glu | 18.61 | 0.0638 | 0.986 | 20.42 | 0.1010 | 0.992 |
| Go-Fe3O4@SiO2@mSiO2-Glu | 20.96 | 0.0641 | 0.988 | 22.91 | 0.1022 | 0.991 |
| Adsorbent | First-Order Model Fitting | Second-Order Model Fitting | ||||
|---|---|---|---|---|---|---|
| Qe,cal/mg/g | k1 | R2 | Qe,cal/mg/g | k2 | R2 | |
| Go-Fe3O4@SiO2@mSiO2-Glu | 23.78 | 0.0621 | 0.966 | 26.14 | 0.0905 | 0.994 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Wang, X.; Dong, M.; Huang, X.; Wu, Z.; Li, J. Magnetic Separation of Oxoacid of Boron from Salt-Lake Brine by Synergistically Enhanced Boron Adsorbents of Glucose-Functionalized SiO2 and Graphene. Int. J. Mol. Sci. 2022, 23, 11356. https://doi.org/10.3390/ijms231911356
Luo Q, Wang X, Dong M, Huang X, Wu Z, Li J. Magnetic Separation of Oxoacid of Boron from Salt-Lake Brine by Synergistically Enhanced Boron Adsorbents of Glucose-Functionalized SiO2 and Graphene. International Journal of Molecular Sciences. 2022; 23(19):11356. https://doi.org/10.3390/ijms231911356
Chicago/Turabian StyleLuo, Qinglong, Xueying Wang, Mingzhe Dong, Xueli Huang, Zhijian Wu, and Jun Li. 2022. "Magnetic Separation of Oxoacid of Boron from Salt-Lake Brine by Synergistically Enhanced Boron Adsorbents of Glucose-Functionalized SiO2 and Graphene" International Journal of Molecular Sciences 23, no. 19: 11356. https://doi.org/10.3390/ijms231911356
APA StyleLuo, Q., Wang, X., Dong, M., Huang, X., Wu, Z., & Li, J. (2022). Magnetic Separation of Oxoacid of Boron from Salt-Lake Brine by Synergistically Enhanced Boron Adsorbents of Glucose-Functionalized SiO2 and Graphene. International Journal of Molecular Sciences, 23(19), 11356. https://doi.org/10.3390/ijms231911356