The Ketogenic Diet Improves Gut–Brain Axis in a Rat Model of Irritable Bowel Syndrome: Impact on 5-HT and BDNF Systems
Abstract
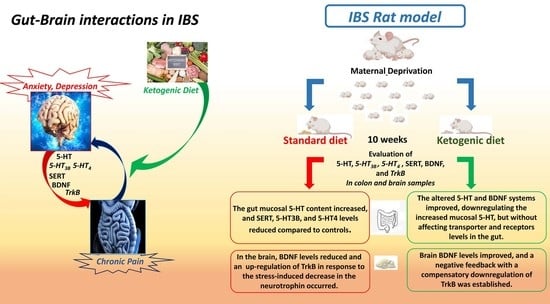
:1. Introduction
2. Results
2.1. Serotonin Levels
2.2. 5-HT System
2.3. BDNF System
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Serotonin Levels
4.3. Western Immunoblotting
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sperber, A.D.; Dumitrascu, D.; Fukudo, S.; Gerson, C.; Ghoshal, U.C.; Gwee, K.A.; Hungin, A.P.S.; Kang, J.Y.; Minhu, C.; Schmulson, M.; et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome Foundation working team literature review. Gut 2017, 66, 1075–1082. [Google Scholar] [CrossRef]
- Ladabaum, U.; Boyd, E.; Zhao, W.K.; Mannalithara, A.; Sharabidze, A.; Singh, G.; Chung, E.; Levin, T.R. Diagnosis, comorbidities, and management of irritable bowel syndrome in patients in a large health maintenance organization. Clin. Gastroenterol. Hepatol. 2012, 10, 37–45. [Google Scholar] [CrossRef]
- Yu, Y.C.; Li, J.; Zhang, M.; Pan, J.C.; Yu, Y.; Zhang, J.B.; Zheng, L.; Si, J.M.; Xu, Y. Resveratrol Improves Brain-Gut Axis by Regulation of 5-HT-Dependent Signaling in the Rat Model of Irritable Bowel Syndrome. Front. Cell Neurosci. 2019, 13, 30. [Google Scholar] [CrossRef]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable Bowel Syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef] [PubMed]
- Grifka-Walk, H.M.; Jenkins, B.R.; Kominsky, D.J. Amino Acid Trp: The Far Out Impacts of Host and Commensal Tryptophan Metabolism. Front. Immunol. 2021, 12, 653208. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.B.; Hsiao, E.Y. Roles for the gut microbiota in regulating neuronal feeding circuits. J. Clin. Investig. 2021, 131, 131. [Google Scholar] [CrossRef] [PubMed]
- Diwakarla, S.; Fothergill, L.J.; Fakhry, J.; Callaghan, B.; Furness, J.B. Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neurogastroenterol. Motil. 2017, 29, e13101. [Google Scholar] [CrossRef] [PubMed]
- Shajib, M.S.; Khan, W.I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2015, 213, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Skadhauge, E. Signal transduction pathways for serotonin as an intestinal secretagogue. Comp. Biochem. Physiol. A Physiol. 1997, 118, 283–290. [Google Scholar] [CrossRef]
- Stasi, C.; Bellini, M.; Bassotti, G.; Blandizzi, C.; Milani, S. Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Tech. Coloproctol. 2014, 18, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D. Review article: Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment. Pharmacol. Ther. 1999, 13 (Suppl. S2), 15–30. [Google Scholar] [CrossRef]
- Coleman, N.S.; Marciani, L.; Blackshaw, E.; Wright, J.; Parker, M.; Yano, T.; Yamazaki, S.; Chan, P.Q.; Wilde, K.; Gowland, P.A.; et al. Effect of a novel 5-HT3 receptor agonist MKC-733 on upper gastrointestinal motility in humans. Aliment. Pharmacol. Ther. 2003, 18, 1039–1048. [Google Scholar] [CrossRef]
- Talley, N.J. Serotoninergic neuroenteric modulators. Lancet 2001, 358, 2061–2068. [Google Scholar] [CrossRef]
- Morita, H.; Mochiki, E.; Takahashi, N.; Kawamura, K.; Watanabe, A.; Sutou, T.; Ogawa, A.; Yanai, M.; Ogata, K.; Fujii, T.; et al. Effects of 5-HT2B, 5-HT3 and 5-HT4 receptor antagonists on gastrointestinal motor activity in dogs. World J. Gastroenterol. 2013, 19, 6604–6612. [Google Scholar] [CrossRef]
- Vahora, I.S.; Tsouklidis, N.; Kumar, R.; Soni, R.; Khan, S. How Serotonin Level Fluctuation Affects the Effectiveness of Treatment in Irritable Bowel Syndrome. Cureus 2020, 12, e9871. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Guo, Y.T.; Gao, J.P.; Liu, J.J.; Chang, Z.P.; Feng, X.J.; Xu, D.; Deng, G.F.; Hou, R.G. Shaoyao-Gancao Decoction Relieves Visceral Hyperalgesia in TNBS-Induced Postinflammatory Irritable Bowel Syndrome via Inactivating Transient Receptor Potential Vanilloid Type 1 and Reducing Serotonin Synthesis. Evid. Based Complement. Altern. Med. 2020, 2020, 7830280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, Y.; Wang, E.; Meng, Y.; Bi, Z.; Sun, S.; Zhang, C.; Fan, H.; Yuan, J. Shugan Decoction Alleviates Colonic Dysmotility in Female SERT-Knockout Rats by Decreasing M3 Receptor Expression. Front. Pharmacol. 2020, 11, 01082. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cui, H.; Li, T.; Qi, J.; Chen, H.; Gao, F.; Tian, X.; Mu, Y.; He, R.; Lv, S.; et al. Synergistic Effect of Berberine-Based Chinese Medicine Assembled Nanostructures on Diarrhea-Predominant Irritable Bowel Syndrome In Vivo. Front. Pharmacol. 2020, 11, 1210. [Google Scholar] [CrossRef]
- Arevalo, J.C.; Wu, S.H. Neurotrophin signaling: Many exciting surprises! Cell Mol. Life Sci. 2006, 63, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zeng, Y.; Yang, W.; Wu, J. Irritable bowel syndrome may be induced by decreased neuroplasticity. Neuro Endocrinol. Lett. 2014, 35, 655–665. [Google Scholar] [PubMed]
- Lommatzsch, M.; Braun, A.; Mannsfeldt, A.; Botchkarev, V.A.; Botchkareva, N.V.; Paus, R.; Fischer, A.; Lewin, G.R.; Renz, H. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived Neurotrophic functions. Am. J. Pathol. 1999, 155, 1183–1193. [Google Scholar] [CrossRef]
- Liu, S. Neurotrophic factors in enteric physiology and pathophysiology. Neurogastroenterol. Motil. 2018, 30, e13446. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Chimienti, G.; Riezzo, G.; Linsalata, M.; D’Attoma, B.; Clemente, C.; Orlando, A. Adipose Tissue-Derived Biomarkers of Intestinal Barrier Functions for the Characterization of Diarrhoea-Predominant IBS. Dis. Mark. 2018, 2018, 1827937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, G.; Liu, D.R.; Wang, Y.; Yao, S.K. Increased expression of brain-derived neurotrophic factor is correlated with visceral hypersensitivity in patients with diarrhea-predominant irritable bowel syndrome. World J. Gastroenterol. 2019, 25, 269–281. [Google Scholar] [CrossRef]
- Wang, P.; Du, C.; Chen, F.X.; Li, C.Q.; Yu, Y.B.; Han, T.; Akhtar, S.; Zuo, X.L.; Tan, X.D.; Li, Y.Q. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci. Rep. 2016, 6, 20320. [Google Scholar] [CrossRef]
- Konturek, T.J.; Martinez, C.; Niesler, B.; van der Voort, I.; Monnikes, H.; Stengel, A.; Goebel-Stengel, M. The Role of Brain-Derived Neurotrophic Factor in Irritable Bowel Syndrome. Front. Psychiatry 2020, 11, 531385. [Google Scholar] [CrossRef]
- Fan, F.; Tang, Y.; Dai, H.; Cao, Y.; Sun, P.; Chen, Y.; Chen, A.; Lin, C. Blockade of BDNF signalling attenuates chronic visceral hypersensitivity in an IBS-like rat model. Eur. J. Pain 2020, 24, 839–850. [Google Scholar] [CrossRef]
- Yu, Z.C.; Cen, Y.X.; Wu, B.H.; Wei, C.; Xiong, F.; Li, D.F.; Liu, T.T.; Luo, M.H.; Guo, L.L.; Li, Y.X.; et al. Berberine prevents stress-induced gut inflammation and visceral hypersensitivity and reduces intestinal motility in rats. World J. Gastroenterol. 2019, 25, 3956–3971. [Google Scholar] [CrossRef]
- Patel, N.V. “Let Food Be Thy Medicine”: Diet and Supplements in Irritable Bowel Syndrome. Clin. Exp. Gastroenterol. 2021, 14, 377–384. [Google Scholar] [CrossRef]
- Field, R.; Field, T.; Pourkazemi, F.; Rooney, K. Ketogenic diets and the nervous system: A scoping review of neurological outcomes from nutritional ketosis in animal studies. Nutr. Res. Rev. 2021, 1–14. [Google Scholar] [CrossRef]
- Yarar-Fisher, C.; Li, J.; Womack, E.D.; Alharbi, A.; Seira, O.; Kolehmainen, K.L.; Plunet, W.T.; Alaeiilkhchi, N.; Tetzlaff, W. Ketogenic regimens for acute neurotraumatic events. Curr. Opin. Biotechnol. 2021, 70, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G.; Evangelista, S. Experimental Models of Irritable Bowel Syndrome and the Role of the Enteric Neurotransmission. J. Clin. Med. 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Reddel, S.; Putignani, L.; Del Chierico, F. The Impact of Low-FODMAPs, Gluten-Free, and Ketoenic Diets on Gut Microbiota Modulation in Pathological Conditions. Nutrients 2019, 11, 373. [Google Scholar] [CrossRef]
- Gigante, I.; Tutino, V.; Russo, F.; De Nunzio, V.; Coletta, S.; Armentano, R.; Crovace, A.; Caruso, M.G.; Orlando, A.; Notarnicola, M. Cannabinoid Receptors Overexpression in a Rat Model of Irritable Bowel Syndrome (IBS) after Treatment with a Ketogenic Diet. Int. J. Mol. Sci. 2021, 22, 2880. [Google Scholar] [CrossRef]
- Chimienti, G.; Orlando, A.; Lezza, A.M.S.; D’Attoma, B.; Notarnicola, M.; Gigante, I.; Pesce, V.; Russo, F. The Ketogenic Diet Reduces the Harmful Effects of Stress on Gut Mitochondrial Biogenesis in a Rat Model of Irritable Bowel Syndrome. Int. J. Mol. Sci. 2021, 22, 3498. [Google Scholar] [CrossRef]
- Lutas, A.; Yellen, G. The ketogenic diet: Metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013, 36, 32–40. [Google Scholar] [CrossRef]
- Zilberter, T.; Zil-berter, Y. Ketogenic Ratio Determines Metabolic Effects of Macronutrients and Prevents Interpretive Bias. Front. Nutr. 2018, 5, 75. [Google Scholar] [CrossRef]
- Camilleri, M.; Andrews, C.N.; Bharucha, A.E.; Carlson, P.J.; Ferber, I.; Stephens, D.; Smyrk, T.C.; Urrutia, R.; Aerssens, J.; Thielemans, L.; et al. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology 2007, 132, 17–25. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, M.; Dou, D.; Kang, T.; Li, F. Effects of Deoxyschisandrin on Visceral Sensitivity of Mice with Inflammatory Bowel Disease. Evid. Based Complement. Altern. Med. 2019, 2019, 2986097. [Google Scholar] [CrossRef]
- Otani, K.; Okada, M.; Yamawaki, H. Diverse distribution of tyrosine receptor kinase B isoforms in rat multiple tissues. J. Vet. Med. Sci. 2017, 79, 1516–1523. [Google Scholar] [CrossRef]
- Nibuya, M.; Takahashi, M.; Russell, D.S.; Duman, R.S. Repeated stress increases catalytic TrkB mRNA in rat hippocampus. Neurosci. Lett. 1999, 267, 81–84. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.M.; Hakkarainen, A.; Lehtimäki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Järvinen, H. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7347–7354. [Google Scholar] [CrossRef]
- Lane, J.; Brown, N.I.; Williams, S.; Plaisance, E.P.; Fontaine, K.R. Ketogenic Diet for Cancer: Critical Assessment and Research Recommendations. Nutrients 2021, 13, 3562. [Google Scholar] [CrossRef]
- Operto, F.F.; Matricardi, S.; Pastorino, G.M.G.; Verrotti, A.; Coppola, G. The Ketogenic Diet for the Treatment of Mood Disorders in Comorbidity with Epilepsy in Children and Adolescents. Front. Pharmacol. 2020, 11, 578396. [Google Scholar] [CrossRef]
- Araya, A.V.; Orellana, X.; Espinoza, J. Evaluation of the effect of caloric restriction on serum BDNF in overweight and obese subjects: Preliminary evidences. Endocrine 2008, 33, 300–304. [Google Scholar] [CrossRef]
- Russo, F.; Chimienti, G.; Clemente, C.; Ferreri, C.; Orlando, A.; Riezzo, G. A possible role for ghrelin, leptin, brain-derived neurotrophic factor and docosahexaenoic acid in reducing the quality of life of coeliac disease patients following a gluten-free diet. Eur. J. Nutr. 2017, 56, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Gyorkos, A.; Baker, M.H.; Miutz, L.N.; Lown, D.A.; Jones, M.A.; Houghton-Rahrig, L.D. Carbohydrate-restricted Diet and Exercise Increase Brain-derived Neurotrophic Factor and Cognitive Function: A Randomized Crossover Trial. Cureus 2019, 11, e5604. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kwak, Y.S. Effect of nutrients and exhaustive exercise on brain function. J. Exerc. Rehabil. 2019, 15, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Seguella, L.; Pesce, M.; Capuano, R.; Casano, F.; Pesce, M.; Corpetti, C.; Vincenzi, M.; Maftei, D.; Lattanzi, R.; Del Re, A.; et al. High-fat diet impairs duodenal barrier function and elicits glia-dependent changes along the gut-brain axis that are required for anxiogenic and depressive-like behaviors. J. Neuroinflamm. 2021, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Iacovides, S.; Goble, D.; Paterson, B.; Meiring, R.M. Three consecutive weeks of nutritional ketosis has no effect on cognitive function, sleep, and mood compared with a high-carbohydrate, low-fat diet in healthy individuals: A randomized, crossover, controlled trial. Am. J. Clin. Nutr. 2019, 110, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Brownlow, M.L.; Jung, S.H.; Moore, R.J.; Bechmann, N.; Jankord, R. Nutritional Ketosis Affects Metabolism and Behavior in Sprague-Dawley Rats in Both Control and Chronic Stress Environments. Front. Mol. Neurosci. 2017, 10, 129. [Google Scholar] [CrossRef] [PubMed]





| Group | Rats (Number) | Maternal Deprivation (3 h/Day from PNDs 2 to 14) | Treatment (for Ten Weeks after PND 14) |
|---|---|---|---|
| Ctrl-Std | 12 | No | Standard diet |
| Ctrl-KD | 13 | No | Ketogenic diet |
| IBS-Std | 11 | Yes | Standard diet |
| IBS-KD | 17 | Yes | Ketogenic diet |
| Analytical Constituents | Standard Diet | Ketogenic Diet |
|---|---|---|
| Moisture | 12% | 0% |
| Crude protein | 18.5% | 16.0% |
| Crude oils and fats | 3.0% | 67.0% |
| Crude fibers | 6.0% | 6.0% |
| Crude ash | 7.0% | 4.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, A.; Chimienti, G.; Notarnicola, M.; Russo, F. The Ketogenic Diet Improves Gut–Brain Axis in a Rat Model of Irritable Bowel Syndrome: Impact on 5-HT and BDNF Systems. Int. J. Mol. Sci. 2022, 23, 1098. https://doi.org/10.3390/ijms23031098
Orlando A, Chimienti G, Notarnicola M, Russo F. The Ketogenic Diet Improves Gut–Brain Axis in a Rat Model of Irritable Bowel Syndrome: Impact on 5-HT and BDNF Systems. International Journal of Molecular Sciences. 2022; 23(3):1098. https://doi.org/10.3390/ijms23031098
Chicago/Turabian StyleOrlando, Antonella, Guglielmina Chimienti, Maria Notarnicola, and Francesco Russo. 2022. "The Ketogenic Diet Improves Gut–Brain Axis in a Rat Model of Irritable Bowel Syndrome: Impact on 5-HT and BDNF Systems" International Journal of Molecular Sciences 23, no. 3: 1098. https://doi.org/10.3390/ijms23031098
APA StyleOrlando, A., Chimienti, G., Notarnicola, M., & Russo, F. (2022). The Ketogenic Diet Improves Gut–Brain Axis in a Rat Model of Irritable Bowel Syndrome: Impact on 5-HT and BDNF Systems. International Journal of Molecular Sciences, 23(3), 1098. https://doi.org/10.3390/ijms23031098