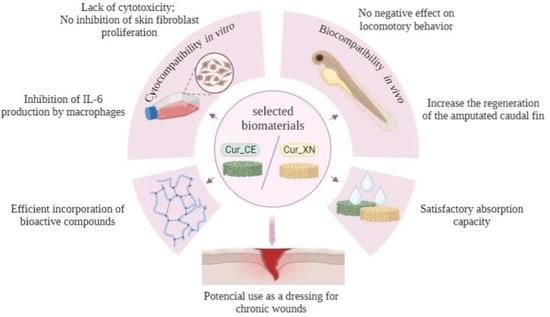
Do Curdlan Hydrogels Improved with Bioactive Compounds from Hop Exhibit Beneficial Properties for Skin Wound Healing?
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant and Anti-Inflammatory Activities of Hop Compounds
2.2. ATR-FTIR Spectra of Biomaterials
2.3. SEM Images and EDS Spectra of Biomaterials
2.4. Contact Angle of Biomaterials
2.5. Swelling Ability of Biomaterials
2.6. Antibacterial Activity of Biomaterials
2.7. Cell Culture Experiments In Vitro
2.7.1. The Influence of Biomaterials on the Viability and Proliferation of Human Fibroblasts
2.7.2. The Influence of Biomaterials on the Production of IL-6 by LPS-Stimulated Human Macrophages
2.8. Preliminary In Vivo Experiments in Zebrafish Larvae Model
2.8.1. The Influence of Biomaterials on Larval Locomotor Activity
2.8.2. The Influence of Biomaterials on Caudal Fin Regeneration
3. Materials and Methods
3.1. Materials
3.2. Characterization of CE and XN
3.3. Preparation of Curdlan-Based Biomaterials Improved with CE or XN
3.4. ATR-FTIR Analysis
3.5. Microstructure Analysis
3.6. Contact Angle Measurements
3.7. Evaluation of Wound Exudate Absorption Capacity
3.8. Evaluation of Cell-Biomaterial Interaction In Vitro
3.8.1. Evaluation of Antibacterial Properties
3.8.2. Cytocompatibility towards Human Skin Fibroblasts
3.8.3. Inflammatory Activity of Human Macrophages
3.9. Evaluation of Response of Zebrafish (Danio rerio) Larvae to Biomaterials
3.9.1. Locomotor Behavior
3.9.2. Regeneration Process
3.10. Statistical Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Nurzynska, A.; Klimek, K.; Swierzycka, I.; Palka, K.; Ginalska, G. Porous curdlan-based hydrogels modified with copper ions as potential dressings for prevention and management of bacterial wound infection—An in vitro assessment. Polymers 2020, 12, 1893. [Google Scholar] [CrossRef]
- Nurzynska, A.; Klimek, K.; Palka, K.; Szajnecki, Ł.; Ginalska, G. Curdlan-based hydrogels for potential application as dressings for promotion of skin wound healing-preliminary in vitro studies. Materials 2021, 14, 2344. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; De Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef] [Green Version]
- Michalicha, A.; Roguska, A.; Przekora, A.; Budzyńska, B.; Belcarz, A. Poly(levodopa)-modified β-glucan as a candidate for wound dressings. Carbohydr. Polym. 2021, 272, 118485. [Google Scholar] [CrossRef]
- Wojcik, M.; Kazimierczak, P.; Benko, A.; Palka, K.; Vivcharenko, V.; Przekora, A. Superabsorbent curdlan-based foam dressings with typical hydrocolloinds properties for highly exuding wound management. Mater. Sci. Eng. C 2021, 124, 112068. [Google Scholar] [CrossRef]
- Trinh, X.-T.; Long, N.-V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.-Y.; Heo, C.-Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef]
- Majtan, J.; Jesenak, M. β-Glucans: Multi-Functional Modulator of Wound Healing. Molecules 2018, 23, 806. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Qi, X.; Cai, E.; Zhang, C.; Wang, J.; Lan, Y.; Deng, H.; Shen, J.; Hu, R. Highly efficient bacteria-infected diabetic wound healing employing a melanin-reinforced biopolymer hydrogel. Chem. Eng. J. 2023, 460, 141852. [Google Scholar] [CrossRef]
- Muthuramalingam, K.; Choi, S.I.; Hyun, C.; Kim, Y.M.; Cho, M. β-Glucan-Based Wet Dressing for Cutaneous Wound Healing. Adv. Wound Care 2019, 8, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pino, P.; Pellegrino, G.; Ronchetti, S.; Mollea, C.; Bosco, F.; Onida, B. Antibacterial β-Glucan/Zinc Oxide Nanocomposite Films for Wound Healing. Bionanoscience 2023, 13, 426–435. [Google Scholar] [CrossRef]
- Wojcik, M.; Kazimierczak, P.; Belcarz, A.; Wilczynska, A.; Vivcharenko, V.; Pajchel, L.; Adaszek, L.; Przekora, A. Biocompatible curdlan-based biomaterials loaded with gentamicin and Zn-doped nano-hydroxyapatite as promising dressing materials for the treatment of infected wounds and prevention of surgical site infections. Biomater. Adv. 2022, 139, 213006. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Qi, X.; Mao, R.; Pan, W.; Zhang, M.; Wu, X.; Chen, G.; Shen, J.; Deng, H.; Hu, R. Construction of functional curdlan hydrogels with bio-inspired polydopamine for synergistic periodontal antibacterial therapeutics. Carbohydr. Polym. 2020, 245, 116585. [Google Scholar] [CrossRef]
- Ponticelli, M.; Russo, D.; Faraone, I.; Sinisgalli, C.; Labanca, F.; Lela, L.; Milella, L. The promising ability of Humulus lupulus L. Iso-α-acids vs. diabetes, inflammation, and metabolic syndrome: A systematic review. Molecules 2021, 26, 954. [Google Scholar] [CrossRef]
- Chen, W.J.; Lin, J.K. Mechanisms of Cancer Chemoprevention by Hop Bitter Acids (Beer Aroma) through Induction of Apoptosis Mediated by Fas and Caspase Cascades. J. Agric. Food Chem. 2004, 52, 55–64. [Google Scholar] [CrossRef]
- Rój, E.; Tadić, V.M.; Mišić, D.; Žižović, I.; Arsić, I.; Dobrzyńska-Inger, A.; Kostrzewa, D. Supercritical carbon dioxide hops extracts with antimicrobial properties. Open Chem. 2015, 13, 1157–1171. [Google Scholar] [CrossRef]
- Sleha, R.; Radochova, V.; Malis, J.; Mikyska, A.; Houska, M.; Krofta, K.; Bogdanova, K.; Janovska, S.; Pejchal, J.; Kolar, M.; et al. Strong antimicrobial and healing effects of beta-acids from hops in methicillin-resistant Staphylococcus aureus-infected external wounds in vivo. Antibiotics 2021, 10, 708. [Google Scholar] [CrossRef]
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from Hop: Hope for cancer prevention and treatment. IUBMB Life 2021, 73, 1016–1044. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Haque, E.; Śmiech, M.; Taniguchi, H.; Jamieson, S.; Tewari, D.; Bishayee, A. Xanthohumol for human malignancies: Chemistry, pharmacokinetics and molecular targets. Int. J. Mol. Sci. 2021, 22, 4478. [Google Scholar] [CrossRef] [PubMed]
- Klimek, K.; Tyśkiewicz, K.; Miazga-Karska, M.; Dębczak, A.; Rój, E.; Ginalska, G. Bioactive compounds obtained from polish “marynka” hop variety using efficient two-step supercritical fluid extraction and comparison of their antibacterial, cytotoxic, and anti-proliferative activities in vitro. Molecules 2021, 26, 2366. [Google Scholar] [CrossRef]
- Paszkot, J.; Kawa-Rygielska, J.; Anioł, M. Properties of dry hopped dark beers with high xanthohumol content. Antioxidants 2021, 10, 763. [Google Scholar] [CrossRef]
- Sławińska-Brych, A.; Zdzisińska, B.; Czerwonka, A.; Mizerska-Kowalska, M.; Dmoszyńska-Graniczka, M.; Stepulak, A.; Gagoś, M. Xanthohumol exhibits anti-myeloma activity in vitro through inhibition of cell proliferation, induction of apoptosis via the ERK and JNK-dependent mechanism, and suppression of sIL-6R and VEGF production. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129408. [Google Scholar] [CrossRef] [PubMed]
- Negrão, R.; Costa, R.; Duarte, D.; Gomes, T.T.; Coelho, P.; Guimarães, J.T.; Guardão, L.; Azevedo, I.; Soares, R. Xanthohumol-supplemented beer modulates angiogenesis and inflammation in a skin wound healing model. Involvement of local adipocytes. J. Cell. Biochem. 2012, 113, 100–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sychrová, A.; Škovranová, G.; Čulenová, M.; Bittner Fialová, S. Prenylated Flavonoids in Topical Infections and Wound Healing. Molecules 2022, 27, 4491. [Google Scholar] [CrossRef]
- Costa, R.; Negrão, R.; Valente, I.; Castela, A.; Duarte, D.; Guardão, L.; Magalhães, P.J.; Rodrigues, J.A.; Guimarães, J.T.; Gomes, P.; et al. Xanthohumol modulates inflammation, oxidative stress, and angiogenesis in type 1 diabetic rat skin wound healing. J. Nat. Prod. 2013, 76, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, M.; Dong, H.; Miao, J.; Stagos, D.; Liu, M. Dietary prenylated flavonoid xanthohumol alleviates oxidatove damage and acceleretes diabetic wound healing via Nrf2 activation. Food Chem. Toxicol. 2022, 160, 112813. [Google Scholar] [CrossRef]
- Tian, B.; Xu, D.; Cheng, J.; Liu, Y. Chitosan-silica with hops β-acids added films as prospective food packaging materials: Preparation, characterization, and properties. Carbohydr. Polym. 2021, 272, 118457. [Google Scholar] [CrossRef] [PubMed]
- Bajić, M.; Jalšovec, H.; Travan, A.; Novak, U.; Likozar, B. Chitosan-based films with incorporated supercritical CO2 hop extract: Structural, physicochemical, and antibacterial properties. Carbohydr. Polym. 2019, 219, 261–268. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling antibiotic resistance with compounds of natural origin: A comprehensive review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef]
- Asokan, G.V.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO global priority pathogens list: A bibliometric analysis of medline-pubmed for knowledge mobilization to infection prevention and control practices in Bahrain. Oman Med. J. 2019, 34, 184–193. [Google Scholar] [CrossRef]
- Zhang, R.; Edgar, K.J. Properties, chemistry, and applications of the bioactive polysaccharide curdlan. Biomacromolecules 2014, 15, 1079–1096. [Google Scholar] [CrossRef]
- Velot, É.; Ducrocq, F.; Girardeau, L.; Hehn, A.; Piutti, S.; Kahn, C.; Linder, M.; Bianchi, A.; Arab-Tehrany, E. Hop Extract Anti-Inflammatory Effect on Human Chondrocytes Is Potentiated When Encapsulated in Rapeseed Lecithin Nanoliposomes. Int. J. Mol. Sci. 2022, 23, 12423. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Kim, K.-Y.; Lee, S.-H.; Kim, M.-Y.; Park, H.-J.; Jeong, H.-S. Antioxidant and Antitumor Activities of Methanolic Extracts from Humulus japonicus. Korean J. Food Nutr. 2012, 25, 357–361. [Google Scholar] [CrossRef] [Green Version]
- Lyu, J., II; Ryu, J.; Seo, K.; Kang, K.; Park, S.H.; Ha, T.H.; Ahn, J.; Kang, S. Comparative Study on Phenolic Compounds and Antioxidant Activities of Hop (Humulus lupulus L.) Strobile Extracts. Plants 2022, 11, 135. [Google Scholar] [CrossRef]
- Dybowski, M.P.; Klimek, K.; Typek, R.; Miazga-Karska, M.; Krzemi, B. The Anti-Acne Potential and Chemical Composition of Two Cultivated Cotoneaster Species. Cells 2022, 11, 367. [Google Scholar]
- Carbone, K.; Gervasi, F. An Updated Review of the Genus Humulus: A Valuable Source of Bioactive Compounds for Health and Disease Prevention. Plants 2022, 11, 3434. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cho, B.O.; Shin, J.Y.; Hao, S.; Jang, S.I. Humulus japonicus extract alleviates oxidative stress and apoptosis in 6-hydroxydopamine-induced PC12 cells. Asian Pac. J. Trop. Biomed. 2022, 12, 197. [Google Scholar]
- Kontek, B.; Jedrejek, D.; Oleszek, W.; Olas, B. Antiradical and antioxidant activity in vitro of hops-derived extracts rich in bitter acids and xanthohumol. Ind. Crops Prod. 2021, 161, 113208. [Google Scholar] [CrossRef]
- Van Cleemput, M.; Cattoor, K.; De Bosscher, K.; Haegeman, G.; De Keukeleire, D.; Heyerick, A. Hop (Humulus lupulus)-Derived Bitter Acids as Multipotent Bioactive Compounds. J. Nat. Prod. 2009, 72, 1220–1230. [Google Scholar] [CrossRef]
- Philips, N.; Samuel, M.; Arena, R.; Chen, Y.J.; Conte, J.; Natarajan, P.; Haas, G.; Gon-Zales, S. Direct inhibition of elastase and matrixmetalloproteinases and stimulation of biosynthesis of fibrillar collagens, elastin, and fibrillins by xanthohumol. J. Cosmet. Sci. 2010, 61, 485. [Google Scholar] [CrossRef]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef]
- Kurahashi, T.; Fujii, J. Roles of Antioxidative Enzymes in Wound Healing. J. Dev. Biol. 2015, 3, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Kosmalska, A.; Zaborski, M. Characteristics of compounds in hops using cyclic voltammetry, UV–VIS, FTIR and GC–MS analysis. Food Chem. 2014, 156, 353–361. [Google Scholar] [CrossRef]
- Arczewska, M.; Kami, D.M.; Górecka, E.; Pociecha, D.; Rój, E.; Adrianna, S.; Gago, M. The molecular organization of prenylated flavonoid xanthohumol in DPPC multibilayers: X-ray diffraction and FTIR spectroscopic studies. Biochim. Biophys. Acta 2013, 1828, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Hong, T.; Yin, J.; Nie, S.; Xie, M. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, C.; Thaiane, T.; Carvalho, V.; Sato, F.; Matioli, G. Use of FT-IR, FT-Raman and thermal analysis to evaluate the gel formation of curdlan produced by Agrobacterium sp. IFO 13140 and determination of its rheological properties with food applicability. Food Chem. 2017, 232, 369–378. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, H.; Yin, Y.; Nishinari, K. Comparison of curdlan and its carboxymethylated derivative by means of Rheology, DSC, and AFM. Carbohydr. Res. 2006, 341, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Shi, W.; Luo, M.; Jiang, F.; Zhang, B. Polyvinyl alcohol inclusion can optimize the sol-gel, mechanical and hydrophobic features of agar/konjac glucomannan system. Carbohydr. Polym. 2022, 277, 118879. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Li, H.; Shi, W.; Lu, J.; Zhang, L.; Zhang, B. Increasing agar content improves the sol-gel and mechanical features of starch/agar binary system. Carbohydr. Polym. 2022, 278, 118906. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Nirmal, N.P.; Chuprom, J. Blend film based on fish gelatine/curdlan for packaging applications: Spectral, microstructural and thermal characteristics. RSC Adv. 2015, 5, 99044–99057. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, T.; Zhang, Y.; Zhang, C.; Lu, Z.; Lu, F.; Zhao, H. Effect of Tea Polyphenols on Curdlan/Chitosan Blending Film Properties and Its Application to Chilled Meat Preservation. Coatings 2019, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xu, L.; Ma, J.; Ye, Y.; Chen, Y.; Qian, J. Introduction of Curdlan Optimizes the Comprehensive Properties of Methyl Cellulose Films. Foods 2023, 12, 547. [Google Scholar] [CrossRef]
- Permyakova, E.S.; Konopatsky, A.S.; Ershov, K.I.; Bakhareva, K.I.; Sitnikova, N.A.; Shtansky, D.V.; Solovieva, A.O.; Manakhov, A.M. Ag-Contained Superabsorbent Curdlan–Chitosan Foams for Healing Wounds in a Type-2 Diabetic Mice Model. Pharmaceutics 2022, 14, 724. [Google Scholar] [CrossRef]
- Budziak, I.; Arczewska, M.; Kamiński, D.M. Formation of Prenylated Chalcone Xanthohumol Cocrystals: Single Crystal X-ray Diffraction, Vibrational Spectroscopic Study Coupled with Multivariate Analysis. Molecules 2019, 24, 4245. [Google Scholar] [CrossRef] [Green Version]
- Klimek, K.; Ginalska, G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications—A Review. Polymers 2020, 12, 844. [Google Scholar] [CrossRef] [Green Version]
- Bartmańska, A.; Wałecka-Zacharska, E.; Tronina, T.; Popłoński, J.; Sordon, S.; Brzezowska, E.; Bania, J.; Huszcza, E. Antimicrobial Properties of Spent Hops Extracts, Flavonoids Isolated Therefrom, and Their Derivatives. Molecules 2018, 23, 2059. [Google Scholar] [CrossRef] [Green Version]
- Michiu, D.; Delvigne, F.; Mabon, N.; Jimborean, M.; Fogarasi, M.; Mihai, M.; Tofană, M.; Thonart, P. Inhibitory Effects of Iso-α and β Hop Acids Against Pediococcus pentosaceus. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1316–1322. [Google Scholar] [CrossRef] [Green Version]
- Cermak, P.; Olsovska, J.; Mikyska, A.; Dusek, M.; Kadleckova, Z.; Vanicek, J.; Nyc, O.; Sigler, K.; Bostikova, V.; Bostik, P. Strong antimicrobial activity of xanthohumol and other derivatives from hops (Humulus lupulus L.) on gut anaerobic bacteria. APMIS 2017, 125, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Frank, U.; Schempp, C.M.; Wölfle, U. Hop Extract Acts as an Antioxidant with Antimicrobial Effects against Propionibacterium Acnes and Staphylococcus Aureus. Molecules 2019, 24, 223. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial biomaterials for skin wound dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in Antibacterial Hydrogel Dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef]
- Cömert Önder, F.; Ay, M.; Aydoğan Türkoğlu, S.; Tura Köçkar, F.; Çelik, A. Antiproliferative activity of Humulus lupulus extracts on human hepatoma (Hep3B), colon (HT-29) cancer cells and proteases, tyrosinase, β-lactamase enzyme inhibition studies. J. Enzyme Inhib. Med. Chem. 2016, 31, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, D.O.; Freitas, J.; Nogueira, P.; Henriques, S.N.; Carmo, A.M.; Castro, M.A.; Guido, L.F. Xanthohumol inhibits cell proliferation and induces apoptosis in human thyroid cells. Food Chem. Toxicol. 2018, 121, 450–457. [Google Scholar] [CrossRef]
- Yoo, Y.B.; Park, K.S.; Kim, J.B.; Kang, H.J.; Yang, J.H.; Lee, E.K.; Kim, H.Y. Xanthohumol inhibits cellular proliferation in a breast cancer cell line (MDA-MB231) through an intrinsic mitochondrial-dependent pathway. Indian J. Cancer 2014, 51, 518–523. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Wessjohann, L.A. Cytotoxic effect of commercial Humulus lupulus L. (hop) preparations—In comparison to its metabolomic fingerprint. J. Adv. Res. 2013, 4, 417–421. [Google Scholar] [CrossRef] [Green Version]
- Saugspier, M.; Dorn, C.; Czech, B.; Gehrig, M.; Heilmann, J.; Hellerbrand, C. Hop bitter acids inhibit tumorigenicity of hepatocellular carcinoma cells in vitro. Oncol. Rep. 2012, 28, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.Y.; Kim, H.J.; Han, J.S. Anti-inflammatory effects of calcium citrate in RAW 264.7cells via suppression of NF-κB activation. Environ. Toxicol. Pharmacol. 2015, 39, 27–34. [Google Scholar] [CrossRef]
- Desousa, J.; Tong, M.; Wei, J.; Chamley, L.; Stone, P.; Chen, Q. The anti-inflammatory effect of calcium for preventing endothelial cell activation in preeclampsia. J. Hum. Hypertens. 2016, 30, 303–308. [Google Scholar] [CrossRef]
- Karnad, A.; Patil, P.; Majagi, S. Calcium enhances antiinflammatory activity of aspirin in albino rats. Indian J. Pharmacol. 2006, 38, 397–402. [Google Scholar]
- van Cleemput, M.; Heyerick, A.; Libert, C.; Swerts, K.; Philippé, J.; de Keukeleire, D.; Haegeman, G.; de Bosscher, K. Hop bitter acids efficiently block inflammation independent of GRα, PPARα, or PPARγ. Mol. Nutr. Food Res. 2009, 53, 1143–1155. [Google Scholar] [CrossRef]
- Vazquez-Cervantes, G.I.; Ortega, D.R.; Ayala, T.B.; de la Cruz, V.P.; Esquivel, D.F.G.; Salazar, A.; Pineda, B. Redox and anti-inflammatory properties from hop components in beer-related to neuroprotection. Nutrients 2021, 13, 2000. [Google Scholar] [CrossRef]
- Wu, C.N.; Sun, L.C.; Chu, Y.L.; Yu, R.C.; Hsieh, C.W.; Hsu, H.Y.; Hsu, F.C.; Cheng, K.C. Bioactive compounds with anti-oxidative and anti-inflammatory activities of hop extracts. Food Chem. 2020, 330, 127244. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Lim, J.; Gal, J.; Kang, J.C.; Kim, H.J.; Kang, B.Y.; Choi, H.J. Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem. Int. 2011, 58, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Tian, L.; Gao, G.; Peng, S.; Zhang, J.; Wu, D.; Huang, J.; Hua, Q.; Lu, T.; Zhong, L.; et al. Inhibitory effects of polystyrene microplastics on caudal fin regeneration in zebrafish larvae. Environ. Pollut. 2020, 266, 114664. [Google Scholar] [CrossRef] [PubMed]
- Sojan, J.M.; Gioacchini, G.; Giorgini, E.; Orlando, P.; Tiano, L. Zebrafish caudal fin as a model to investigate the role of probiotics in bone regeneration. Sci. Rep. 2022, 12, 8057. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult Zebrafish as a Model System for Cutaneous Wound-Healing Research. J. Investig. Dermatol. 2013, 133, 1655–1665. [Google Scholar] [CrossRef] [Green Version]
- Naomi, R.; Bahari, H.; Yazid, M.D.; Embong, H. Zebrafish as a Model System to Study the Mechanism of Cutaneous Wound Healing and Drug Discovery: Advantages and Challenges. Pharmaceuticals 2021, 14, 1058. [Google Scholar] [CrossRef] [PubMed]
- Lisse, T.S.; Brochu, E.A.; Rieger, S. Capturing Tissue Repair in Zebrafish Larvae with Time-lapse Brightfield Stereomicroscopy. J. Vis. Exp. 2015, 95, e52654. [Google Scholar] [CrossRef] [Green Version]
- Chávez, M.N.; Aedo, G.; Fierro, F.A.; Allende, M.L.; Egaña, J.T. Zebrafish as an Emerging Model Organism to Study Angiogenesis in Development and Regeneration. Front. Physiol. 2016, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- Xie, Y.; Meijer, A.H.; Schaaf, M.J.M. Modeling Inflammation in Zebrafish for the Development of Anti-inflammatory Drugs. Front. Cell Dev. Biol. 2021, 8, 620984. [Google Scholar] [CrossRef]
- Raghupathy, S.; Vaidyanathan, L.; Sivaswamy, L.T.S. Adult Zebrafish Model of Wound Inflammation to Study Wound Healing Potency of Curcuma longa Extracts. Annu. Res. Rev. Biol. 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Kang, M.; Lee, H.H.L.; Cho, C.H.; Choi, I.; Park, Y.; Lee, S. Antioxidant Effects of Turmeric Leaf Extract against Hydrogen Peroxide-Induced Oxidative Stress In Vitro in Vero Cells and In Vivo in Zebrafish. Antioxidants 2021, 10, 112. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standarization: Genewa, Switzerland, 2009.
- ISO 10993-12:2012; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standarization: Genewa, Switzerland, 2007.
- Nurzynska, A.; Piotrowski, P.; Klimek, K.; Król, J.; Kaim, A.; Ginalska, G. Novel C60 Fullerenol-Gentamicin Conjugate–Physicochemical Characterization and Evaluation of Antibacterial and Cytotoxic Properties. Molecules 2022, 27, 4366. [Google Scholar] [CrossRef] [PubMed]
- Rangaraj, S.; Venkatachalam, R. In vitro and in vivo characteristics of biogenic high surface silica nanoparticles in A549 lung cancer cell lines and Danio rerio model systems for inorganic biomaterials development. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- Makkar, H.; Verma, S.K.; Panda, P.K.; Jha, E.; Das, B.; Mukherjee, K.; Suar, M. In Vivo Molecular Toxicity Profile of Dental Bioceramics in Embryonic Zebrafish (Danio rerio). Chem. Res. Toxicol. 2018, 31, 914–923. [Google Scholar] [CrossRef]
- Witherel, C.E.; Gurevich, D.; Collin, J.D.; Martin, P.; Spiller, K.L. Host-Biomaterial Interactions in Zebrafish. ACS Biomater. Sci. Eng. 2018, 4, 1233–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rácz, A.; Allan, B.; Dwyer, T.; Thambithurai, D.; Crespel, A.; Killen, S.S. Identification of individual zebrafish (Danio rerio): A refined protocol for vie tagging whilst considering animal welfare and the principles of the 3rs. Animals 2021, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, V. Zebrafish: A Practical Approach. Genet. Res. 2003, 82, 79. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef] [PubMed]











| Biomaterial Code | Concentration of Curdlan (Cur) [wt.%] | Concentration of Crude Extract (CE) [μg/mL] | Concentration of Xanthohumol (XN) [μg/mL] |
|---|---|---|---|
| Cur | 11 | - | - |
| Cur_CE_0.125 | 11 | 0.125 | - |
| Cur_CE_0.25 | 11 | 0.25 | - |
| Cur_CE_0.5 | 11 | 0.5 | - |
| Cur_CE_1 | 11 | 1 | - |
| Cur_CE_2 | 11 | 2 | - |
| Cur_CE_4 | 11 | 4 | - |
| Cur_CE_8 | 11 | 8 | - |
| Cur_CE_16 | 11 | 16 | - |
| Cur_XN_0.0156 | 11 | - | 0.0156 |
| Cur_XN_0.0313 | 11 | - | 0.03123 |
| Cur_XN_0.0625 | 11 | - | 0.0625 |
| Cur_XN_0.125 | 11 | - | 0.125 |
| Cur_XN_0.25 | 11 | - | 0.25 |
| Cur_XN_0.5 | 11 | - | 0.5 |
| Cur_XN_1 | 11 | - | 1 |
| Cur_XN_2 | 11 | - | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurzynska, A.; Klimek, K.; Michalak, A.; Dos Santos Szewczyk, K.; Arczewska, M.; Szalaj, U.; Gagos, M.; Ginalska, G. Do Curdlan Hydrogels Improved with Bioactive Compounds from Hop Exhibit Beneficial Properties for Skin Wound Healing? Int. J. Mol. Sci. 2023, 24, 10295. https://doi.org/10.3390/ijms241210295
Nurzynska A, Klimek K, Michalak A, Dos Santos Szewczyk K, Arczewska M, Szalaj U, Gagos M, Ginalska G. Do Curdlan Hydrogels Improved with Bioactive Compounds from Hop Exhibit Beneficial Properties for Skin Wound Healing? International Journal of Molecular Sciences. 2023; 24(12):10295. https://doi.org/10.3390/ijms241210295
Chicago/Turabian StyleNurzynska, Aleksandra, Katarzyna Klimek, Agnieszka Michalak, Katarzyna Dos Santos Szewczyk, Marta Arczewska, Urszula Szalaj, Mariusz Gagos, and Grazyna Ginalska. 2023. "Do Curdlan Hydrogels Improved with Bioactive Compounds from Hop Exhibit Beneficial Properties for Skin Wound Healing?" International Journal of Molecular Sciences 24, no. 12: 10295. https://doi.org/10.3390/ijms241210295
APA StyleNurzynska, A., Klimek, K., Michalak, A., Dos Santos Szewczyk, K., Arczewska, M., Szalaj, U., Gagos, M., & Ginalska, G. (2023). Do Curdlan Hydrogels Improved with Bioactive Compounds from Hop Exhibit Beneficial Properties for Skin Wound Healing? International Journal of Molecular Sciences, 24(12), 10295. https://doi.org/10.3390/ijms241210295