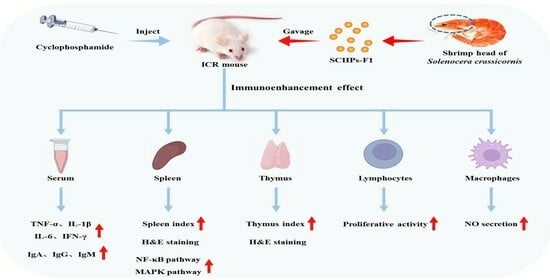
Effects of Low Molecular Weight Peptides from Red Shrimp (Solenocera crassicornis) Head on Immune Response in Immunosuppressed Mice
Abstract
:1. Introduction
2. Results
2.1. Characterization of SCHPs-F1
2.2. Effect of SCHPs-F1 on Body Weight and Immune Organ Index
2.3. Effects on Histological Changes of the Spleen and Thymus
2.4. Effect of SCHPs-F1 on the Proliferation of Spleen Lymphocytes
2.5. Effect of SCHPs-F1 on NO Secretion in Peritoneal Macrophages
2.6. Effects of SCHPs-F1 on Cytokine Production
2.7. Effects of SCHPs-F1 on Humoral Immunity
2.8. Effects of SCHPs-F1 on the NF-κB and MAPK Pathway in the Spleen
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animal Treatment
4.3. Analysis of Body Weight and Immune Organ Index
4.4. Histomorphology
4.5. Splenic Lymphocyte Proliferation Assay
4.6. Measurement of NO Production
4.7. Assay of Cytokines and Immunoglobulins in Serum
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cota, A.M.; Midwinter, M.J. The immune system. Anaesth. Intensive Care Med. 2012, 13, 273–275. [Google Scholar] [CrossRef]
- Martí, A.; Marcos, A.; Martínez, J.A. Obesity and immune function relationships. Obes. Rev. 2001, 2, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Petrie, K.J.; Booth, R.J.; Elder, H.; Cameron, L.D. Psychological influences on the perception of immune function. Psychol. Med. 1999, 29, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Farrar, J.D. Adrenergic regulation of immune cell function and inflammation. Semin. Immunopathol. 2020, 42, 709–717. [Google Scholar] [CrossRef]
- Liu, Y.-W.J.; Mutnuri, S.; Siddiqui, S.B.; Weikle, G.R.; Oladipo, O.; Ganti, N.; Beach, R.E.; Afrouzian, M. Levamisole-adulterated cocaine nephrotoxicity: Ultrastructural features. Am. J. Clin. Pathol. 2016, 145, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Durymanov, M.; Permyakova, A.; Reineke, J. Pre-treatment with PLGA/Silibinin nanoparticles mitigates dacarbazine-induced hepatotoxicity. Front. Bioeng. Biotechnol. 2020, 8, 495. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.H.; Jo, S.; Cho, M.J.; Cho, Y.R.; Lee, Y.J.; Byun, S. Immunomodulatory functional foods and their molecular mechanisms. Exp. Mol. Med. 2022, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Yang, X.; Chen, J.; Peng, T.; Yin, X.; Liu, W.; Liang, M.; Wan, J.; Yang, X. Antioxidative effects and mechanism study of bioactive peptides from defatted walnut (Juglans regia L.) meal hydrolysate. J. Agric. Food Chem. 2019, 67, 3305–3312. [Google Scholar] [CrossRef]
- Bravo, F.I.; Mas-Capdevila, A.; López-Fernández-Sobrino, R.; Torres-Fuentes, C.; Mulero, M.; Alcaide-Hidalgo, J.M.; Muguerza, B. Identification of novel antihypertensive peptides from wine lees hydrolysate. Food Chem. 2022, 366, 130690. [Google Scholar] [CrossRef]
- Díaz-Gómez, J.L.; Castorena-Torres, F.; Preciado-Ortiz, R.E.; García-Lara, S. Anti-cancer activity of maize bioactive peptides. Front. Chem. 2017, 5, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Ren, L.; Zhang, L.; Qiao, Q.; Farooq, M.Z.; Xu, Q. The potential of food protein-derived bioactive peptides against chronic intestinal inflammation. Mediat. Inflamm. 2020, 2020, 6817156. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.; Ho, T.C.; Ahmed, R.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Chun, B.-S. Biofunctional properties of bacterial collagenolytic protease-extracted collagen hydrolysates obtained using catalysts-assisted subcritical water hydrolysis. J. Ind. Eng. Chem. 2020, 81, 332–339. [Google Scholar] [CrossRef]
- Bechaux, J.; Gatellier, P.; Le Page, J.-F.; Drillet, Y.; Sante-Lhoutellier, V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct. 2019, 10, 6244–6266. [Google Scholar] [CrossRef] [PubMed]
- Suraiya, S.; Ahmmed, M.K.; Haq, M. Immunity boosting roles of biofunctional compounds available in aquafoods: A review. Heliyon 2022, 8, e09547. [Google Scholar] [CrossRef]
- Hou, H.; Fan, Y.; Li, B.; Xue, C.; Yu, G.; Zhang, Z.; Zhao, X. Purification and identification of immunomodulating peptides from enzymatic hydrolysates of Alaska pollock frame. Food Chem. 2012, 134, 821–828. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, K.-B.-W.-R.; Sung, N.-Y.; Byun, E.-H.; Nam, H.-S.; Ahn, D.-H. Immune-enhancement effects of tuna cooking drip and its enzymatic hydrolysate in Balb/c mice. Food Sci. Biotechnol. 2018, 27, 131–137. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Z.; Ye, S.; Hong, X.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G. Immunoenhancement effects of pentadecapeptide derived from Cyclina sinensis on immune-deficient mice induced by cyclophosphamide. J. Funct. Foods 2019, 60, 103408. [Google Scholar] [CrossRef]
- Xu, Y.; Sui, J.; Ma, L.; Dong, D.; Kou, Q.; Gan, Z.; Gong, L.; Yang, M.; Li, X.; Wang, J.; et al. Spatial pattern of benthic macroinvertebrate communities and their relationship with environmental variables on the East China Sea shelf. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 169–170, 104633. [Google Scholar] [CrossRef]
- Song, R.; Jia, Z.; Xu, Y.; Zhang, X.; Wei, R.; Sun, J. Saponification to improve the antioxidant activity of astaxanthin extracts from Penaeus sinensis (Solenocera crassicornis) by-products and intervention effect on Paracetamol-induced acute hepatic injury in rat. J. Funct. Foods 2020, 73, 104150. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Z.; Yu, F.; Zhang, Z.; Yang, Z.; Tang, Y.; Ding, G. Ameliorative effect of low molecular weight peptides from the head of red shrimp (Solenocera crassicornis) against cyclophosphamide-induced hepatotoxicity in mice. J. Funct. Foods 2020, 72, 104085. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Z.; Huang, F.; Yang, Z.; Yu, F.; Tang, Y.; Ding, G. Protective effect of low molecular weight peptides from Solenocera crassicornis head against cyclophosphamide-induced nephrotoxicity in mice via the Keap1/Nrf2 pathway. Antioxidants 2020, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cheng, Q.; Peng, Q.; Yu, X.; Yin, X.; Liang, M.; Ma, C.W.; Huang, Z.; Jia, W. Antioxidant peptides derived from the hydrolyzate of purple sea urchin (Strongylocentrotus nudus) gonad alleviate oxidative stress in Caenorhabditis elegans. J. Funct. Foods 2018, 48, 594–604. [Google Scholar] [CrossRef]
- Zheng, K.; Li, Q.; Lin, D.; Zong, X.; Luo, X.; Yang, M.; Yue, X.; Ma, S. Peptidomic analysis of pilose antler and its inhibitory effect on triple-negative breast cancer at multiple sites. Food Funct. 2020, 11, 7481–7494. [Google Scholar] [CrossRef] [PubMed]
- Adu-Berchie, K.; Mooney, D.J. Biomaterials as local niches for immunomodulation. Acc. Chem. Res. 2020, 53, 1749–1760. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Liu, X.; Pan, W.; Li, N.; Tang, B. Intelligent stimuli-responsive nano immunomodulators for cancer immunotherapy. Chem. Sci. 2021, 12, 3130–3145. [Google Scholar] [CrossRef]
- Alsaffar, R.M.; Ali, S.; Rashid, S.; Rashid, S.M.; Majid, S.; Rehman, M.U. Immunomodulation: An immune regulatory mechanism in carcinoma therapeutics. Int. Immunopharmacol. 2021, 99, 107984. [Google Scholar] [CrossRef]
- Santiago-López, L.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Mata-Haro, V.; González-Córdova, A.F. Food-derived immunomodulatory peptides. J. Sci. Food Agric. 2016, 96, 3631–3641. [Google Scholar] [CrossRef]
- Yu, F.; He, K.; Dong, X.; Zhang, Z.; Wang, F.; Tang, Y.; Chen, Y.; Ding, G. Immunomodulatory activity of low molecular-weight peptides from Nibea japonica skin in cyclophosphamide-induced immunosuppressed mice. J. Funct. Foods 2020, 68, 103888. [Google Scholar] [CrossRef]
- He, K.; Zeng, Y.; Tian, H.; Zhang, Z.; Zhang, H.; Huang, F.; Yu, F. Macrophage immunomodulatory effects of low molecular weight peptides from Mytilus coruscus via NF-κB/MAPK signaling pathways. J. Funct. Foods 2021, 83, 104562. [Google Scholar] [CrossRef]
- Moignet, A.; Hasanali, Z.; Zambello, R.; Pavan, L.; Bareau, B.; Tournilhac, O.; Roussel, M.; Fest, T.; Awwad, A.; Baab, K.; et al. Cyclophosphamide as a first-line therapy in LGL leukemia. Leukemia 2014, 28, 1134–1136. [Google Scholar] [CrossRef]
- Pondugula, S.R.; Harshan, A.; Ramesh, S.; Govindarajulu, M.; Almaghrabi, M.; Majrashi, M.; Abbott, K.L.; Nadar, R.; Alturki, M.; Salamat, J.M.; et al. Cardioprotective effects of Oroxylum indicum extract against doxorubicin and cyclophosphamide-induced cardiotoxicity. Cardiovasc. Toxicol. 2022, 22, 67–77. [Google Scholar] [CrossRef]
- Li, W.-J.; Li, L.; Zhen, W.-Y.; Wang, L.-F.; Pan, M.; Lv, J.-Q.; Wang, F.; Yao, Y.-F.; Nie, S.-P.; Xie, M.-Y. Ganoderma atrum polysaccharide ameliorates ROS generation and apoptosis in spleen and thymus of immunosuppressed mice. Food Chem. Toxicol. 2017, 99, 199–208. [Google Scholar] [CrossRef]
- Heinzel, S.; Marchingo, J.M.; Horton, M.B.; Hodgkin, P.D. The regulation of lymphocyte activation and proliferation. Curr. Opin. Immunol. 2018, 51, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.; Pacher, P.; Haskó, G. Role of macrophages in the endocrine system. Trends Endocrinol. Metab. 2021, 32, 238–256. [Google Scholar] [CrossRef]
- Cione, E.; Plastina, P.; Pingitore, A.; Perri, M.; Caroleo, M.C.; Fazio, A.; Witkamp, R.; Meijerink, J. Capsaicin analogues derived from n-3 polyunsaturated fatty acids (PUFAs) reduce inflammatory activity of macrophages and stimulate insulin secretion by β-cells in vitro. Nutrients 2019, 11, 915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Ortiz, A.; Serrador, J.M. Nitric oxide signaling in T cell-mediated immunity. Trends Mol. Med. 2018, 24, 412–427. [Google Scholar] [CrossRef]
- Huang, L.; Shen, M.; Wu, T.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Mesona chinensis Benth polysaccharides protect against oxidative stress and immunosuppression in cyclophosphamide-treated mice via MAPKs signal transduction pathways. Int. J. Biol. Macromol. 2020, 152, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X.; Wang, Y.; Jin, W.; Guo, Y. The immunoenhancement effects of starfish Asterias rollestoni polysaccharides in macrophages and cyclophosphamide-induced immunosuppression mouse models. Food Funct. 2020, 11, 10700–10708. [Google Scholar] [CrossRef]
- Tang, Y.; Pu, Q.; Zhao, Q.; Zhou, Y.; Jiang, X.; Han, T. Effects of fucoidan isolated from Laminaria japonica on immune response and gut microbiota in cyclophosphamide-treated mice. Front. Immunol. 2022, 13, 916618. [Google Scholar] [CrossRef]
- Megha, K.B.; Mohanan, P.V. Role of immunoglobulin and antibodies in disease management. Int. J. Biol. Macromol. 2021, 169, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Han, W.; Zhang, J.; Zhang, Z.; Su, X. Advances in the regulatory effects of bioactive peptides on metabolic signaling pathways in tumor cells. J. Cancer 2019, 10, 2425–2433. [Google Scholar] [CrossRef] [Green Version]
- Guha, S.; Majumder, K. Structural-features of food-derived bioactive peptides with anti-inflammatory activity: A brief review. J. Food Biochem. 2019, 43, 12531. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, X.; Xie, L.; Xie, J.; Shen, M. Sulfated Chinese yam polysaccharide enhances the immunomodulatory activity of RAW 264.7 cells via the TLR4-MAPK/NF-κB signaling pathway. Food Funct. 2022, 13, 1316–1326. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.-B.; Shim, J.-H.; Abd El-Aty, A.M. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: A review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Pan, L.; Wu, H.; Zhang, L.; Zhang, Y.; Zhang, Y.; Yang, J.; Lin, Z.; Zhang, M. Isolation, identification, and immunomodulatory mechanism of peptides from Lepidium meyenii (Maca) protein hydrolysate. J. Agric. Food Chem. 2022, 70, 4328–4341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Zhao, R.; Pu, Q.; Jiang, S.; Yu, F.; Yang, Z.; Han, T. Investigation of nephrotoxicity on mice exposed to polystyrene nanoplastics and the potential amelioration effects of DHA-enriched phosphatidylserine. Sci. Total Environ. 2023, 892, 164808. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhao, Y.; Li, Q.; Jiang, S.; Tang, Y.; Han, T. DHA-enriched phosphatidylserine alleviates high fat diet-induced jejunum injury in mice by modulating gut microbiota. Food Funct. 2023, 14, 1415. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Wang, P.; Liu, C.; Wang, J.; Liu, X.; Liu, J.; Min, W. Hazelnut protein-derived peptide LDAPGHR shows anti-inflammatory activity on LPS-induced RAW264.7 macrophage. J. Funct. Foods 2018, 46, 449–455. [Google Scholar] [CrossRef]
- Arana-Argáez, V.E.; Mena-Rejón, G.J.; Torres-Romero, J.C.; Lara-Riegos, J.C.; López-Mirón, G.; Carballo, R.M. Anti-inflammatory effects of Chrysophyllum cainito fruit extract in lipopolysaccharide-stimulated mouse peritoneal macrophages. Inflammopharmacology 2021, 29, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Luo, J.; Du, H.; Jiang, Y.; Tu, Y.; Yao, Y.; Xu, M. Ovotransferrin enhances intestinal immune response in cyclophosphamide-induced immunosuppressed mice. Int. J. Biol. Macromol. 2018, 120, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Tian, S.; Jiang, S.; Tang, Y.; Han, T. DHA-enriched phosphatidylcholine from Clupea harengus roes regulates the gut–liver axis to ameliorate high-fat diet-induced non-alcoholic fatty liver disease. Food Funct. 2022, 13, 11555–11567. [Google Scholar] [CrossRef] [PubMed]











| Group | The Spleen Index (mg/g) | The Thymus Index (mg/g) |
|---|---|---|
| Control | 4.48 ± 1.55 | 2.35 ± 0.80 |
| Model | 2.79 ± 1.14 * | 1.86 ± 0.53 ** |
| SCHPs-F1 100 | 3.78 ± 0.50 | 1.93 ± 0.28 * |
| SCHPs-F1 200 | 4.14 ± 1.37 + | 2.04 ± 0.42 + |
| SCHPs-F1 400 | 4.44 ± 0.78 ++ | 2.14 ± 0.60 ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Jiang, S.; Tang, Y.; Ding, G. Effects of Low Molecular Weight Peptides from Red Shrimp (Solenocera crassicornis) Head on Immune Response in Immunosuppressed Mice. Int. J. Mol. Sci. 2023, 24, 10297. https://doi.org/10.3390/ijms241210297
Zhao R, Jiang S, Tang Y, Ding G. Effects of Low Molecular Weight Peptides from Red Shrimp (Solenocera crassicornis) Head on Immune Response in Immunosuppressed Mice. International Journal of Molecular Sciences. 2023; 24(12):10297. https://doi.org/10.3390/ijms241210297
Chicago/Turabian StyleZhao, Rui, Shuoqi Jiang, Yunping Tang, and Guofang Ding. 2023. "Effects of Low Molecular Weight Peptides from Red Shrimp (Solenocera crassicornis) Head on Immune Response in Immunosuppressed Mice" International Journal of Molecular Sciences 24, no. 12: 10297. https://doi.org/10.3390/ijms241210297
APA StyleZhao, R., Jiang, S., Tang, Y., & Ding, G. (2023). Effects of Low Molecular Weight Peptides from Red Shrimp (Solenocera crassicornis) Head on Immune Response in Immunosuppressed Mice. International Journal of Molecular Sciences, 24(12), 10297. https://doi.org/10.3390/ijms241210297