Pyrolysis Temperature vs. Application Rate of Biochar Amendments: Impacts on Soil Microbiota and Metribuzin Degradation
Abstract
:1. Introduction
2. Results
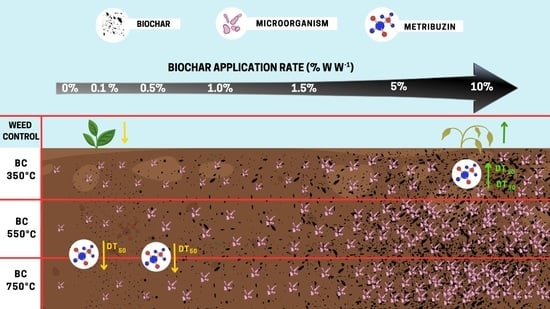
2.1. Metribuzin Degradation
2.2. Respiration Rate of the Microbial Rhizosphere
3. Discussion
4. Materials and Methods
4.1. Soil Collection and Analysis
4.2. Sugarcane Straw Biochar
4.3. Soil Preparation and Application of Metribuzin
4.4. Extraction of Metribuzin
4.5. Chromatographic Conditions
4.6. Degradation Kinetics of Metribuzin in Soil
4.7. Respiration Rate of the Microbial Rhizosphere
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mielke, K.C.; Mendes, K.F.; Sousa, R.N.; Medeiros, B.A.P. Degradation Process of Herbicides in Biochar-Amended Soils: Impact on Persistence and Remediation. In Biodegradation Technology of Organic and Inorganic Pollutants; Mendes, K.F., Sousa, R.N., Mielke, K.C., Eds.; IntechOpen: Rijeka, Croatia, 2022; pp. 1–23. [Google Scholar]
- Mehdizadeh, M.; Mushtaq, W.; Siddiqui, S.A.; Ayadi, S.; Kaur, P.; Yeboah, S.; Mazraedoost, S.; AL-Taey, D.K.A.; Tampubolon, K. Herbicide residues in agroecosystems: Fate, detection, and effect on non-target plants. Rev. Agric. Sci. 2021, 9, 157–167. [Google Scholar] [CrossRef]
- Tudararo-Aherobo, L.E.; Ataikiru, T.L. Effects of chronic use of herbicides on soil physicochemical and microbiological characteristics. Microbiol. Res. J. Int. 2020, 30, 9–19. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Cunill, J.M. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 2833. [Google Scholar] [CrossRef] [PubMed]
- Pateiro-Moure, M.; Arias-Estévez, M.; Simal-Gándara, J. Critical review on the environmental fate of quaternary ammonium herbicides in soils devoted to vineyards. Environ. Sci. Technol. 2013, 47, 4984–4998. [Google Scholar] [CrossRef]
- Mendes, K.F.; Mielke, K.C.; Júnior, L.H.B.; de la Cruz, R.A.; Sousa, R.N. Anaerobic and aerobic degradation studies of herbicides and radiorespirometry of microbial activity in soil. In Radioisotopes in Weed Research; Mendes, K.F., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 95–125. [Google Scholar]
- Guimarães, A.C.D.; Inoue, M.H.; Mendes, K.F.; Santos, A.W.; Oliveira, A.M. Processo de Degradação Biológica (Biodegradação) dos Herbicidas no Solo. In Herbicidas no Ambiente: Comportamento e Destino; Mendes, K.F., Inoue, M.H., Tornisielo, V.L., Eds.; Editora UFV: Viçosa, Brazil, 2022; pp. 9–35. [Google Scholar]
- Meng, X.; Guo, Y.; Wang, Y.; Fan, S.; Wang, K.; Han, W. A Systematic Review of Photolysis and Hydrolysis Degradation Modes, Degradation Mechanisms, and Identification Methods of Pesticides. J. Chem. 2022, 2022, 9552466. [Google Scholar] [CrossRef]
- Łozowicka, B.; Wołejko, E.; Kaczyński, P.; Konecki, R.; Iwaniuk, P.; Drągowski, W.; Jablońska-Trypuć, A. Effect of microorganism on behaviour of two commonly used herbicides in wheat/soil system. Appl. Soil Ecol. 2021, 162, 103879. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management: Science; Lehmann, J., Joseph, S., Eds.; Technology e Implementation; Routledge: New York, NY, USA, 2015; pp. 1–13. [Google Scholar]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef]
- Ameloot, N.; Graber, E.R.; Verheijen, F.G.; De Neve, S. Interactions between biochar stability and soil organisms: Review and research needs. Eur. J. Soil Sci. 2013, 64, 379–390. [Google Scholar] [CrossRef]
- López-Piñeiro, A.; Peña, D.; Albarrán, A.; Becerra, D.; Sánchez-Llerena, J. Sorption, leaching and persistence of metribuzin in Mediterranean soils amended with olive mill waste of different degrees of organic matter maturity. J. Environ. Manag. 2013, 122, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.C.C.; Mendes, K.F.; Tornisielo, V.L.; Teófilo, T.M.S.; Takeshita, V.; das Chagas, P.S.F.; Silva, D.V. Effect of pyrolysis temperature on eucalyptus wood residues biochar on availability and transport of hexazinone in soil. Int. J. Environ. Sci. Technol. 2021, 19, 499–514. [Google Scholar] [CrossRef]
- Wu, C.; Liu, X.; Wu, X.; Dong, F.; Xu, J.; Zheng, Y. Sorption, degradation and bioavailability of oxyfluorfen in biochar-amended soils. Sci. Total Environ. 2019, 658, 87–94. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Borchard, N.; Zajkoska, P.; Fernández-Bayo, J.D.; Martinazzo, R.; Berns, A.E.; Burauel, P. Biochar-mediated [14C] atrazine mineralization in atrazine-adapted soils from Belgium and Brazil. J. Agric. Food Chem. 2013, 61, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, V.; de Oliveira, F.S.; Munhoz-Garcia, G.V.; Fernandes, B.C.C.; da Silva Teófilo, T.M.; Mendes, K.F.; Tornisielo, V.L. Effects of Biochar on Degradation Herbicides in the Soil. In Interactions of Biochar and Herbicides in the Environment; Mendes, K.F., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 189–218. [Google Scholar]
- Hashem, A.; Collins, R.M.; Bowran, D.G. Efficacy of interrow weed control techniques in wide row narrow-leaf lupin. Weed Technol. 2011, 25, 135–140. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rezaei, M.; Tseng, T.M.; Mastinu, A. Effects of metribuzin herbicide on some morpho-physiological characteristics of two Echinacea species. Horticulturae 2022, 8, 169. [Google Scholar] [CrossRef]
- Saritha, J.D.; Ramprakash, T.; Rao, P.C.; Madhavi, M. Persistence of metribuzin in tomato growing soils and tomato fruits. Nat. Environm. Pollut. Technol. 2017, 16, 505. [Google Scholar]
- Guimarães, A.C.D.; Mendes, K.F.; dos Reis, F.C.; Campion, T.F.; Christoffoleti, P.J.; Tornisielo, V.L. Role of Soil Physicochemical Properties in Quantifying the Fate of Diuron, Hexazinone, and Metribuzin. Environ. Sci. Pollut. Res. 2018, 25, 12419–12433. [Google Scholar] [CrossRef] [PubMed]
- PPDB—Pesticide Properties Database. Footprint: Creating Tools for Pesticide Risk Assessment and Management in Europe. Metribuzin. Developed by the Agriculture & Environment Research Unit (AERU), University of Hertfordshire, Funded by UK National Sources and the EU-Funded FOOTPRINT Project (FP6-SSP-022704). Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/469.htm (accessed on 18 April 2023).
- Loffredo, E.; Parlavecchia, M.; Perri, G.; Gattullo, R. Comparative assessment of metribuzin sorption efficiency of biochar, hydrochar e vermicompost. J. Environ. Sci. Health Part B 2019, 54, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Mielke, K.C.; Laube, A.F.S.; Guimarães, T.; Brochado, M.G.D.S.; Medeiros, B.A.D.P.; Mendes, K.F. Pyrolysis temperature and application rate of sugarcane straw biochar influence sorption and desorption of metribuzin and soil chemical properties. Processes 2022, 10, 1924. [Google Scholar] [CrossRef]
- Abdollahi, K.; Naeini, S.A.M.; Motlagh, M.B.; Ebrahimi, P.; Roshani, G. Evaluation the effect of organic amendments (manure and biochar) on metribuzin herbicide persistence in soil. Appl. Soil Res. 2020, 8, 149–161. [Google Scholar]
- Haskis, P.; Mantzos, N.; Hela, D.; Patakioutas, G.; Konstantinou, I. Effect of biochar on the mobility and photodegradation of metribuzin and metabolites in soil-biochar thin-layer chromatography plates. Int. J. Environ. Anal. Chem. 2019, 99, 310–327. [Google Scholar] [CrossRef]
- Mutua, G.K.; Ngigi, A.N.; Getenga, Z.M. Degradation Characteristics of Metribuzin in Soils within the Nzoia River Drainage Basin, Kenya. Toxicol. Environ. Chem. 2016, 98, 800–813. [Google Scholar]
- Mahanta, K.; Sarma, A.; Deka, J. Metribuzin dissipation pattern in soil and its residue in soil and chilli. Indian J. Weed Sci. 2018, 50, 405–407. [Google Scholar] [CrossRef]
- Takeshita, V.; Carvalho, L.B.; Galhardi, J.A.; Munhoz-Garcia, G.V.; Pimpinato, R.F.; Oliveira, H.C.; Fraceto, L.F. Development of a preemergent nanoherbicide: From efficiency evaluation to the assessment of environmental fate and risks to soil microorganisms. ACS Nanosci. Au 2022, 2, 307–323. [Google Scholar] [CrossRef]
- Benoit, P.; Perceval, J.; Stenrød, M.; Moni, C.; Eklo, O.M.; Barriuso, E.; Kværner, J. Availability and biodegradation of metribuzin in alluvial soils as affected by temperature and soil properties. Weed Res. 2007, 47, 517–526. [Google Scholar] [CrossRef]
- Juhler, R.K.; Henriksen, T.H.; Ernstsen, V.; Vinther, F.P.; Rosenberg, P. Impact of basic soil parameters on pesticide disappearance investigated by multivariate partial least square regression and statistics. J. Environ. Qual. 2008, 37, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.; Coste, C.M.; Kawar, N.S. Degradation of metribuzin in two soil types of Lebanon. J. Environ. Sci. Health B 2006, 41, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Wahla, A.Q.; Iqbal, S.; Anwar, S.; Firdous, S.; Mueller, J.A. Optimizing the metribuzin degrading potential of a novel bacterial consortium based on Taguchi design of experiment. J. Hazard. Mater. 2018, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Rani, G.; Bhullar, M.S. Persistence of metribuzin in aridisols as affected by various abiotic factors and its effect on soil enzymes. Int. J. Environ. Anal. Chem. 2022, 1, 20. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Murtaza, B.; Bibi, I.; Naeem, M.A.; Niazi, N.K. A critical review of different factors governing the fate of pesticides in soil under biochar application. Sci. Total Environ. 2020, 711, 134645. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Min, L.; Ren, C. Biochars change the sorption and degradation of thiacloprid in soil: Insights into chemical and biological mechanisms. Environ. Pollut. 2018, 236, 158–167. [Google Scholar] [CrossRef]
- Yavari, S.; Sapari, N.B.; Malakahmad, A.; Yavari, S. Degradation of imazapic and imazapyr herbicides in the presence of optimized oil palm empty fruit bunch and rice husk biochars in soil. J. Hazard. Mater. 2019, 366, 636–642. [Google Scholar] [CrossRef]
- Alvarez, D.O.; Mendes, K.F.; Tosi, M.; de Souza, L.F.; Cedano, J.C.C.; de Souza Falcao, N.P.; Tornisielo, V.L. Sorption-desorption and biodegradation of sulfometuron-methyl and its effects on the bacterial communities in Amazonian soils amended with aged biochar. Ecotoxicol. Environ. Saf. 2021, 207, 111222. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Mu, C.L.; Gu, C.; Liu, C.; Liu, X.J. Impact of woodchip biochar amendment on the sorption and dissipation of pesticide acetamiprid in agricultural soils. Chemosphere 2011, 85, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Edwards-Jones, G.; Murphy, D.V. Biochar mediated alterations in herbicide breakdown and leaching in soil. Soil Biol. Biochem. 2011, 43, 804–813. [Google Scholar] [CrossRef]
- Muter, O.; Berzins, A.; Strikauska, S.; Pugajeva, I.; Bartkevics, V.; Dobele, G.; Steiner, C. The effects of woodchip-and straw-derived biochars on the persistence of the herbicide 4-chloro-2-methylphenoxyacetic acid (MCPA) in soils. Ecotoxicol. Environ. Saf. 2014, 109, 93–100. [Google Scholar] [CrossRef]
- Zhelezova, A.; Cederlund, H.; Stenström, J. Effect of biochar amendment and ageing on adsorption and degradation of two herbicides. Water Air Soil Pollut. 2017, 228, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kah, M.; Sigmund, G.; Xiao, F.; Hofmann, T. Sorption of ionizable and ionic organic compounds to biochar, activated carbon and other carbonaceous materials. Water Res. 2017, 124, 673–692. [Google Scholar] [CrossRef]
- Sousa, R.N.; Soares, M.B.; dos Santos, F.H.; Leite, C.N.; Mendes, K.F. Interaction mechanisms between biochar and herbicides. In Interactions of Biochar and Herbicides in the Environment; Mendes, K.F., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 79–130. [Google Scholar]
- Frimpong, K.A.; Abban-Baidoo, E.; Marschner, B. Can combined compost and biochar application improve the quality of a highly weathered coastal savanna soil? Heliyon 2021, 7, e07089. [Google Scholar] [CrossRef] [PubMed]
- Mendes, K.F.; Furtado, I.F.; Sousa, R.N.D.; Lima, A.D.C.; Mielke, K.C.; Brochado, M.G.D.S. Cow bonechar decreases indaziflam pre-emergence herbicidal activity in tropical soil. J. Environ. Sci. Health Part B 2021, 56, 532–539. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, X.; Gao, W.; Chen, Y.; Pan, W.; Tang, Y. Effect of biochar on the bioavailability of difenoconazole and microbial community composition in a pesticide-contaminated soil. Appl. Soil Ecol. 2017, 121, 185–192. [Google Scholar] [CrossRef]
- Jenkins, J.R.; Viger, M.; Arnold, E.C.; Harris, Z.M.; Ventura, M.; Miglietta, F.; Taylor, G. Biochar alters the soil microbiome and soil function: Results of next-generation amplicon sequencing across Europe. GCB Bioenergy 2017, 9, 591–612. [Google Scholar] [CrossRef] [Green Version]
- Noyce, G.L.; Winsborough, C.; Fulthorpe, R.; Basiliko, N. The microbiomes and metagenomes of forest biochars. Sci. Rep. 2016, 6, 26425. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kaur, P.; Jain, D.; Bhullar, M.S. In-vitro evaluation of rice straw biochars’ effect on bispyribac-sodium dissipation and microbial activity in soil. Ecotoxicol. Environ. Saf. 2020, 191, 110204. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Jabbarov, Z.; Arora, N.K.; Wirth, S.; Bellingrath-Kimura, S.D. Biochar mitigates effects of pesticides on soil biological activities. Environ. Sustain. 2021, 4, 335–342. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Kumar, A.; Joseph, S.; Tsechansky, L.; Privat, K.; Schreiter, I.J.; Schüth, C.; Graber, E.R. Biochar aging in contaminated soil promotes Zn immobilization due to changes in biochar surface structural and chemical properties. Sci. Total Environ. 2018, 626, 953–961. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef]
- Islam, K.R.; Weil, R.R. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric. Ecosyst. Environ. 2000, 79, 9–16. [Google Scholar] [CrossRef]
- Wardle, D.A.; Ghani, A.A. A critique of the microbial metabolic quotient (qCO2) as a bioindicator of disturbance and ecosystem development. Soil Biol. Biochem. 1995, 27, 1601–1610. [Google Scholar] [CrossRef]
- Lacerda, K.A.P.; Cordeiro, M.A.S.; Verginassi, A.; Salgado, F.H.M.; Paulino, H.B.; Carneiro, M.A.C. Organic carbon, biomass and microbial activity in an Oxisol under different management systems. Amaz. J. Agric. Environm. Sci. 2013, 56, 249–254. [Google Scholar] [CrossRef]
- Kaschuk, G.; Alberton, O.; Hungria, M. Three decades of soil microbial biomass studies in Brazilian ecosystems: Lessons learned about soil quality and indications for improving sustainability. Soil Biol. Biochem. 2010, 42, 1–13. [Google Scholar] [CrossRef]
- Perucci, P.; Dumontet, S.; Bufo, S.A.; Mazzatura, A.; Casucci, C. Effects of organic amendment and herbicide treatment on soil microbial biomass. Biol. Fertil. Soils 2000, 32, 17–23. [Google Scholar] [CrossRef]
- Liao, H.; Li, Y.; Yao, H. Biochar amendment stimulates utilization of plant-derived carbon by soil bacteria in an intercropping system. Front. Microbiol. 2019, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Li, Q.; Zhang, W.; Zhou, G.; Ma, L.; Min, W.; Hou, Z. Effects of biochar on soil microbial community composition and activity in drip-irrigated desert soil. Eur. J. Soil Biol. 2016, 72, 27–34. [Google Scholar] [CrossRef]
- Hardy, B.; Sleutel, S.; Dufey, J.E.; Cornelis, J.T. The long-term effect of biochar on soil microbial abundance, activity and community structure is overwritten by land management. Front. Environ. Sci. 2019, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Hamer, U.; Marschner, B.; Brodowski, S.; Amelung, W. Interactive priming of black carbon and glucose mineralisation. Org. Geochem. 2004, 35, 823–830. [Google Scholar] [CrossRef]
- Mašek, O.; Budarin, V.; Gronnow, M.; Crombie, K.; Brownsort, P.; Fitzpatrick, E.; Hurst, P. Microwave and slow pyrolysis biochar-Comparison of physical and functional properties. J. Anal. Appl. Pyrolysis 2013, 100, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Sam, A.T.; Asuming-Brempong, S.; Nartey, E.K. Microbial activity and metabolic quotient of microbes in soils amended with biochar and contaminated with atrazine and paraquat. Acta Agric. Scand. B Soil Plant Sci. 2017, 67, 492–509. [Google Scholar] [CrossRef]
- Ge, X.; Cao, Y.; Zhou, B.; Wang, X.; Yang, Z.; Li, M.H. Biochar addition increases subsurface soil microbial biomass buft has limited effects on soil CO2 emissions in subtropical moso bamboo plantations. Appl. Soil Eco. 2019, 142, 155–165. [Google Scholar] [CrossRef]
- INMET—Instituto Nacional de Meteorologia. Available online: http://www.inmet.gov.br/portal/ (accessed on 18 April 2022).
- Mehdizadeh, M.; Izadi-Darbandi, E.; Yazdi, M.T.N.P.; Rastgoo, M.; Malaekeh-Nikouei, B.; Nassirli, H. Impacts of different organic amendments on soil degradation and phytotoxicity of metribuzin. Int. J. Recycl. Org. 2019, 8, 113–121. [Google Scholar] [CrossRef] [Green Version]
- ANVISA—Agência Nacional de Vigilância Sanitária. Resolution RDC N°. 166, 24 Jutly 2017. Provides for the Validation of Analytical Methods and Makes Other Provisions. Available online: http://antigo.anvisa.gov.br/documents/10181/2721567/RDC_166_2017_COMP.pdf/d5fb92b3-6c6b-4130-8670-4e3263763401 (accessed on 18 April 2023).
- Ananyeva, N.D.; Susyan, E.A.; Gavrilenko, E.G. Determination of the soil microbial biomass carbon using the method of substrate-induced respiration. Eurasian Soil Sci. 2011, 44, 1215–1221. [Google Scholar] [CrossRef]
- Stotzky, G. Microbial respiration. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Norman, A.G., Ed.; ACS: Madison, WI, USA, 1965; pp. 1550–1572. [Google Scholar]
- Islam, K.R.; Weil, R.R. Microwave irradiation of soil for routine measurement of microbial biomass carbon. Biol. Fertil. Soils 1998, 27, 408–416. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Soil microbial biomass: The eco-physiological approach. Soil Biol. Biochem. 2010, 42, 2039–2043. [Google Scholar] [CrossRef]






| Pyrolysis Temperature/°C | Biochar Application Rate % (w/w) | C0 | k | DT50 | DT90 | p-Value | R2 |
|---|---|---|---|---|---|---|---|
| mg kg−1 | days−1 | Days | Days | ||||
| - | Unamended | 7.22 ± 0.01 a | 0.094 | 7.37 | 24.49 | <0.0001 | 0.989 |
| 350 | 0.1 | 5.61 ± 0.02 | 0.094 | 7.35 | 24.41 | <0.0001 | 0.976 |
| 0.5 | 6.39 ± 0.03 | 0.090 | 7.70 | 25.58 | <0.0001 | 0.948 | |
| 1.0 | 6.91 ± 0.05 | 0.093 | 7.45 | 24.75 | <0.0001 | 0.955 | |
| 1.5 | 7.64 ± 0.01 | 0.103 | 6.72 | 22.35 | <0.0001 | 0.940 | |
| 5.0 | 11.55 ± 0.03 | 0.072 | 9.62 | 31.98 | <0.0001 | 0.962 | |
| 10.0 | 10.89 ± 0.02 | 0.040 | 17.32 | 57.56 | <0.0001 | 0.947 | |
| 550 | 0.1 | 9.02 ± 0.05 | 0.220 | 3.14 | 10.46 | <0.0001 | 0.966 |
| 0.5 | 8.45 ± 0.08 | 0.156 | 4.44 | 14.76 | <0.0001 | 0.991 | |
| 1.0 | 9.40 ± 0.07 | 0.199 | 3.46 | 15.57 | <0.0001 | 0.991 | |
| 1.5 | 5.75 ± 0.01 | 0.134 | 5.17 | 17.18 | <0.0001 | 0.987 | |
| 5.0 | 8.07 ± 0.01 | 0.138 | 5.02 | 16.68 | <0.0001 | 0.991 | |
| 10.0 | 8.63 ± 0.02 | 0.114 | 6.08 | 20.19 | <0.0001 | 0.983 | |
| 750 | 0.1 | 5.22 ± 0.07 | 0.110 | 6.30 | 20.93 | <0.0001 | 0.970 |
| 0.5 | 6.85 ± 0.05 | 0.135 | 5.13 | 17.05 | <0.0001 | 0.972 | |
| 1.0 | 8.18 ± 0.06 | 0.157 | 4.15 | 14.66 | <0.0001 | 0.986 | |
| 1.5 | 6.41 ± 0.02 | 0.114 | 6.08 | 20.19 | <0.0001 | 0.967 | |
| 5.0 | 7.15 ± 0.03 | 0.101 | 6.86 | 22.79 | <0.0001 | 0.960 | |
| 10.0 | 6.04 ± 0.02 | 0.097 | 7.14 | 23.73 | <0.0001 | 0.980 |
| Pyrolysis Temperature/°C | Application Rate % (w/w) | Chemical Attributes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | OC | P | K | Ca | Mg | H + Al | Zn | Fe | Mn | Cu | B | CEC | BS | ||
| H2O | % | mg kg−1 | mmolc kg−1 | mg kg−1 | mmolc kg−1 | % | |||||||||
| - | Unamended | 5.5 | 1.2 | 1.3 | 77.0 | 15.9 | 5.4 | 33.0 | 3.0 | 129.8 | 91.0 | 3.9 | 0.1 | 23.3 | 41.0 |
| 350 | 0.1 | 5.5 | 1.2 | 1.5 | 97.0 | 16.0 | 5.7 | 33.3 | 2.9 | 129.6 | 99.1 | 3.8 | 0.1 | 24.2 | 40.0 |
| 0.5 | 5.5 | 1.2 | 2.0 | 111.0 | 17.9 | 6.5 | 33.0 | 3.1 | 123.6 | 127.0 | 3.6 | 0.1 | 27.8 | 46.0 | |
| 1 | 5.8 | 1.2 | 3.3 | 125.0 | 17.5 | 6.8 | 26.4 | 2.8 | 148.1 | 130.0 | 3.7 | 0.1 | 29.3 | 52.0 | |
| 1.5 | 5.9 | 1.2 | 6.3 | 139.0 | 17.1 | 7.2 | 23.1 | 2.9 | 154.7 | 144.0 | 4.1 | 0.1 | 29.4 | 56.0 | |
| 5 | 6.8 | 1.2 | 10.0 | 240.0 | 17.7 | 8.3 | 13.3 | 2.9 | 234.4 | 155.0 | 3.8 | 0.1 | 36.7 | 73.0 | |
| 10 | 7.2 | 1.2 | 30.0 | 290.0 | 17.4 | 9.6 | 6.6 | 2.8 | 245.5 | 212.0 | 3.6 | 0.1 | 37.1 | 85.0 | |
| 550 | 0.1 | 5.4 | 1.2 | 2.2 | 99.0 | 16.5 | 5.7 | 29.4 | 2.8 | 128.5 | 94.5 | 3.6 | 0.1 | 24.7 | 48.0 |
| 0.5 | 5.6 | 1.2 | 2.7 | 132.0 | 16.2 | 5.8 | 29.7 | 3.1 | 157.4 | 97.9 | 4.1 | 0.1 | 24.8 | 45.0 | |
| 1 | 5.8 | 1.2 | 4.4 | 158.0 | 17.3 | 6.1 | 29.7 | 3.0 | 228.5 | 91.2 | 4.0 | 0.1 | 26.6 | 47.0 | |
| 1.5 | 5.9 | 1.2 | 8.7 | 161.0 | 17.8 | 5.8 | 19.8 | 2.8 | 266.5 | 157.0 | 3.4 | 0.1 | 25.2 | 56.0 | |
| 5 | 7.0 | 1.2 | 15.0 | 250.0 | 17.7 | 7.6 | 9.9 | 2.7 | 273.5 | 183.0 | 3.1 | 0.1 | 33.2 | 77.0 | |
| 10 | 7.3 | 1.3 | 33.0 | 340.0 | 18.1 | 8.4 | 3.3 | 2.9 | 297.5 | 202.0 | 3.6 | 0.1 | 38.5 | 90.0 | |
| 750 | 0.1 | 5.4 | 1.2 | 2.9 | 108.0 | 16.8 | 5.6 | 33.0 | 2.7 | 135.0 | 96.6 | 3.6 | 0.1 | 25.2 | 43.0 |
| 0.5 | 5.5 | 1.2 | 3.7 | 144.0 | 17.4 | 6.8 | 29.7 | 3.0 | 148.8 | 135.0 | 3.9 | 0.1 | 27.6 | 48.0 | |
| 1 | 5.8 | 1.2 | 7.8 | 178.0 | 17.8 | 7.0 | 29.7 | 2.8 | 147.6 | 122.0 | 4.0 | 0.1 | 29.4 | 49.0 | |
| 1.5 | 6.2 | 1.2 | 12.0 | 240.0 | 18.1 | 7.1 | 13.2 | 2.5 | 238.5 | 123.0 | 3.8 | 0.1 | 30.6 | 70.0 | |
| 5 | 7.2 | 1.3 | 55.0 | 500.0 | 19.7 | 9.8 | 3.3 | 2.9 | 267.5 | 177.0 | 3.7 | 0.1 | 39.3 | 92.0 | |
| 10 | 7.6 | 1.4 | 65.0 | 550.0 | 20.0 | 11.1 | 0.0 | 2.9 | 294.5 | 178.0 | 3.8 | 0.1 | 40.6 | 100.0 | |
| Physical attributes (g kg−1) | |||||||||||||||
| Sand | Silt | Clay | Texture class | ||||||||||||
| Soil | Unamended | 500 | 120 | 380 | Sandy clay | ||||||||||
| Pyrolysis Temperature/°C | pH | C | N | Ash | C/N | SSA |
|---|---|---|---|---|---|---|
| H2O | % | - | m2 g−1 | |||
| 350 | 8.6 | 48.7 | 0.832 | 5.0 | 58.51 | 17 |
| 550 | 9.3 | 49.1 | 0.647 | 10.3 | 75.83 | 129 |
| 750 | 9.8 | 59.0 | 0.403 | 11.6 | 146.36 | 223 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielke, K.C.; Brochado, M.G.d.S.; Laube, A.F.S.; Guimarães, T.; Medeiros, B.A.d.P.; Mendes, K.F. Pyrolysis Temperature vs. Application Rate of Biochar Amendments: Impacts on Soil Microbiota and Metribuzin Degradation. Int. J. Mol. Sci. 2023, 24, 11154. https://doi.org/10.3390/ijms241311154
Mielke KC, Brochado MGdS, Laube AFS, Guimarães T, Medeiros BAdP, Mendes KF. Pyrolysis Temperature vs. Application Rate of Biochar Amendments: Impacts on Soil Microbiota and Metribuzin Degradation. International Journal of Molecular Sciences. 2023; 24(13):11154. https://doi.org/10.3390/ijms241311154
Chicago/Turabian StyleMielke, Kamila Cabral, Maura Gabriela da Silva Brochado, Ana Flávia Souza Laube, Tiago Guimarães, Bruna Aparecida de Paula Medeiros, and Kassio Ferreira Mendes. 2023. "Pyrolysis Temperature vs. Application Rate of Biochar Amendments: Impacts on Soil Microbiota and Metribuzin Degradation" International Journal of Molecular Sciences 24, no. 13: 11154. https://doi.org/10.3390/ijms241311154
APA StyleMielke, K. C., Brochado, M. G. d. S., Laube, A. F. S., Guimarães, T., Medeiros, B. A. d. P., & Mendes, K. F. (2023). Pyrolysis Temperature vs. Application Rate of Biochar Amendments: Impacts on Soil Microbiota and Metribuzin Degradation. International Journal of Molecular Sciences, 24(13), 11154. https://doi.org/10.3390/ijms241311154