Adipocyte- and Monocyte-Mediated Vicious Circle of Inflammation and Obesity (Review of Cellular and Molecular Mechanisms)
Abstract
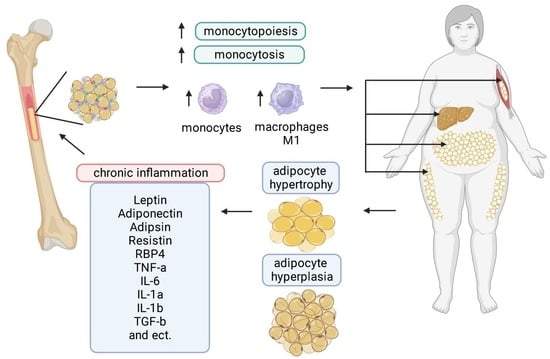
:1. Introduction
2. Adipocytes in Bone Marrow
3. Origin/Formation of Bone Marrow Adipocytes
4. Humoral Factors Affecting Adipogenesis and Myelopoiesis
5. Adipocytes as Factors of the Hematopoietic Microenvironment (HIM)
6. The Role of Bone Marrow Adipocytes in Myelopoiesis in Obesity
7. Additional Factors Affecting Myelopoiesis in Obesity
8. Monocytes
8.1. Characteristics of Monocytes in Metabolic Syndrome (Obesity)
8.2. Activation of Myelopoiesis in Bone Marrow
8.3. Monocytes in Peripheral Blood in Metabolic Syndrome (MS)
8.4. Monocytes/Macrophages in Adipose Tissue in Metabolic Syndrome
8.5. Cells Formed from Bone Marrow Monocytes: Main Players in the Pathogenesis of Metabolic Syndrome
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guha Ray, A.; Odum, O.P.; Wiseman, D.; Weinstock, A. The Diverse Roles of Macrophages in Metabolic Inflammation and Its Resolution. Front. Cell Dev. Biol. 2023, 11, 1147434. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Lavillegrand, J.-R.; Lereverend, C.; Esposito, B.; Cartier, L.; Montabord, M.; Tran-Rajau, J.; Diedisheim, M.; Gruel, N.; Ouguerram, K.; et al. Mild Dyslipidemia Accelerates Tumorigenesis through Expansion of Ly6Chi Monocytes and Differentiation to Pro-Angiogenic Myeloid Cells. Nat. Commun. 2022, 13, 5399. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, Y.; Lee, M.N.; Nah, J.; Yun, N.; Wu, D.; Pae, M. Time-Restricted Feeding Reduces Monocyte Production by Controlling Hematopoietic Stem and Progenitor Cells in the Bone Marrow during Obesity. Front. Immunol. 2022, 13, 1054875. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.; Leite, Â.; Santos, A.; Lima, M.; Barbosa, J.; Cosentino, M.; Ribeiro, L. Predictors of Subclinical Inflammatory Obesity: Plasma Levels of Leptin, Very Low-Density Lipoprotein Cholesterol and CD14 Expression of CD16+ Monocytes. Obes. Facts 2017, 10, 308–322. [Google Scholar] [CrossRef]
- Sharma, M.; Boytard, L.; Hadi, T.; Koelwyn, G.; Simon, R.; Ouimet, M.; Seifert, L.; Spiro, W.; Yan, B.; Hutchison, S.; et al. Enhanced Glycolysis and HIF-1α Activation in Adipose Tissue Macrophages Sustains Local and Systemic Interleukin-1β Production in Obesity. Sci. Rep. 2020, 10, 5555. [Google Scholar] [CrossRef] [Green Version]
- Boroumand, P.; Prescott, D.C.; Mukherjee, T.; Bilan, P.J.; Wong, M.; Shen, J.; Tattoli, I.; Zhou, Y.; Li, A.; Sivasubramaniyam, T.; et al. Bone Marrow Adipocytes Drive the Development of Tissue Invasive Ly6Chigh Monocytes during Obesity. eLife 2022, 11, e65553. [Google Scholar] [CrossRef]
- Coillard, A.; Segura, E. In Vivo Differentiation of Human Monocytes. Front. Immunol. 2019, 10, 1907. [Google Scholar] [CrossRef] [Green Version]
- Corvera, S. Cellular Heterogeneity in Adipose Tissues. Annu. Rev. Physiol. 2021, 83, 257–278. [Google Scholar] [CrossRef]
- Horowitz, M.C.; Berry, R.; Holtrup, B.; Sebo, Z.; Nelson, T.; Fretz, J.A.; Lindskog, D.; Kaplan, J.L.; Ables, G.; Rodeheffer, M.S.; et al. Bone Marrow Adipocytes. Adipocyte 2017, 6, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.L.; Marshall, M.A.; McSkimming, C.C.; Harmon, D.B.; Garmey, J.C.; Oldham, S.N.; Hallowell, P.; McNamara, C.A. Adipocyte Progenitor Cells Initiate Monocyte Chemoattractant Protein-1-Mediated Macrophage Accumulation in Visceral Adipose Tissue. Mol. Metab. 2015, 4, 779–794. [Google Scholar] [CrossRef]
- Ikeda, Y.; Sonoda, N.; Bachuluun, B.; Kimura, S.; Ogawa, Y.; Inoguchi, T. Aberrant Activation of Bone Marrow Ly6C High Monocytes in Diabetic Mice Contributes to Impaired Glucose Tolerance. PLoS ONE 2020, 15, e0229401. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.D.; Tseng, Y.-H. Deciphering Adipose Tissue Heterogeneity. Ann. N. Y. Acad. Sci. 2018, 1411, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, J.; Frazier, T.; Smith, S.; Brown, T.; Bender, R.; McCarthy, M.; Wu, X.; Bunnell, B.A.; Gimble, J.M. Bone Marrow Adipocyte Developmental Origin and Biology. Curr. Osteoporos. Rep. 2018, 16, 312–319. [Google Scholar] [CrossRef]
- De Paula, F.J.A.; Rosen, C.J. Marrow Adipocytes: Origin, Structure, and Function. Annu. Rev. Physiol. 2020, 82, 461–484. [Google Scholar] [CrossRef] [Green Version]
- Suchacki, K.J.; Cawthorn, W.P. Molecular Interaction of Bone Marrow Adipose Tissue with Energy Metabolism. Curr. Mol. Biol. Rep. 2018, 4, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wang, Y.; Zhao, H.; Zhang, H.; Xu, Y.; Wang, S.; Guo, X.; Huang, Y.; Zhang, S.; Han, Y.; et al. Identification and Transcriptome Analysis of Erythroblastic Island Macrophages. Blood 2019, 134, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.; Seu, K.G.; Papoin, J.; Muench, D.E.; Konstantinidis, D.; Olsson, A.; Schlum, K.; Chetal, K.; Chasis, J.A.; Mohandas, N.; et al. Erythroblastic Islands Foster Granulopoiesis in Parallel to Terminal Erythropoiesis. Blood 2022, 140, 1621–1634. [Google Scholar] [CrossRef]
- Heideveld, E.; van den Akker, E. Digesting the Role of Bone Marrow Macrophages on Hematopoiesis. Immunobiology 2017, 222, 814–822. [Google Scholar] [CrossRef]
- Li, Z.; Hardij, J.; Bagchi, D.P.; Scheller, E.L.; MacDougald, O.A. Development, Regulation, Metabolism and Function of Bone Marrow Adipose Tissues. Bone 2018, 110, 134–140. [Google Scholar] [CrossRef]
- Allen, T.D.; Dexter, T.M. Long Term Bone Marrow Cultures: An Ultrastructural Review. Scanning Electron Microsc. 1983, 4, 1851–1866. [Google Scholar]
- Gimble, J.M. The Function of Adipocytes in the Bone Marrow Stroma. New Biol. 1990, 2, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, M. Differential Response of Bone Marrow and Extramedullary Adipose Cells to Starvation. Experientia 1974, 30, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Tratwal, J.; Rojas-Sutterlin, S.; Bataclan, C.; Blum, S.; Naveiras, O. Bone Marrow Adiposity and the Hematopoietic Niche: A Historical Perspective of Reciprocity, Heterogeneity, and Lineage Commitment. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101564. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, S.; Gaculenko, A.; Zhan, Y.; Bozec, A.; Chen, X. Distinct Metabolism of Bone Marrow Adipocytes and Their Role in Bone Metastasis. Front. Endocrinol. 2022, 13, 902033. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.C.; Wenning, L.A.; Miller, W.M.; Papoutsakis, E.T. Modeling PO2 Distributions in the Bone Marrow Hematopoietic Compartment. I. Krogh’s Model. Biophys. J. 2001, 81, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtman, M.A. The Ultrastructure of the Hemopoietic Environment of the Marrow: A Review. Exp. Hematol. 1981, 9, 391–410. [Google Scholar]
- Styner, M.; Pagnotti, G.M.; McGrath, C.; Wu, X.; Sen, B.; Uzer, G.; Xie, Z.; Zong, X.; Styner, M.A.; Rubin, C.T.; et al. Exercise Decreases Marrow Adipose Tissue Through SS-Oxidation in Obese Running Mice. J. Bone Miner. Res. 2017, 32, 1692–1702. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, H.; Wang, S.; Chen, X.; Su, J. Bone Marrow Adipocytes: A Critical Player in the Bone Marrow Microenvironment. Front. Cell Dev. Biol. 2021, 9, 770705. [Google Scholar] [CrossRef]
- Boroumand, P.; Klip, A. Bone Marrow Adipose Cells—Cellular Interactions and Changes with Obesity. J. Cell Sci. 2020, 133, jcs238394. [Google Scholar] [CrossRef] [Green Version]
- Tratwal, J.; Labella, R.; Bravenboer, N.; Kerckhofs, G.; Douni, E.; Scheller, E.L.; Badr, S.; Karampinos, D.C.; Beck-Cormier, S.; Palmisano, B.; et al. Reporting Guidelines, Review of Methodological Standards, and Challenges Toward Harmonization in Bone Marrow Adiposity Research. Report of the Methodologies Working Group of the International Bone Marrow Adiposity Society. Front. Endocrinol. 2020, 11, 65. [Google Scholar] [CrossRef]
- Scheller, E.L.; Doucette, C.R.; Learman, B.S.; Cawthorn, W.P.; Khandaker, S.; Schell, B.; Wu, B.; Ding, S.-Y.; Bredella, M.A.; Fazeli, P.K.; et al. Region-Specific Variation in the Properties of Skeletal Adipocytes Reveals Regulated and Constitutive Marrow Adipose Tissues. Nat. Commun. 2015, 6, 7808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craft, C.S.; Li, Z.; MacDougald, O.A.; Scheller, E.L. Molecular Differences Between Subtypes of Bone Marrow Adipocytes. Curr. Mol. Biol. Rep. 2018, 4, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Gomariz, A.; Helbling, P.M.; Isringhausen, S.; Suessbier, U.; Becker, A.; Boss, A.; Nagasawa, T.; Paul, G.; Goksel, O.; Székely, G.; et al. Quantitative Spatial Analysis of Haematopoiesis-Regulating Stromal Cells in the Bone Marrow Microenvironment by 3D Microscopy. Nat. Commun. 2018, 9, 2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles, H.; Park, S.; Joens, M.S.; Fitzpatrick, J.A.J.; Craft, C.S.; Scheller, E.L. Characterization of the Bone Marrow Adipocyte Niche with Three-Dimensional Electron Microscopy. Bone 2019, 118, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Baryawno, N.; Przybylski, D.; Kowalczyk, M.S.; Kfoury, Y.; Severe, N.; Gustafsson, K.; Kokkaliaris, K.D.; Mercier, F.; Tabaka, M.; Hofree, M.; et al. A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell 2019, 177, 1915–1932.e16. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-Receptor-Expressing Mesenchymal Stromal Cells Represent the Main Source of Bone Formed by Adult Bone Marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef] [Green Version]
- Gasparrini, M.; Rivas, D.; Elbaz, A.; Duque, G. Differential Expression of Cytokines in Subcutaneous and Marrow Fat of Aging C57BL/6J Mice. Exp. Gerontol. 2009, 44, 613–618. [Google Scholar] [CrossRef]
- Tikhonova, A.N.; Dolgalev, I.; Hu, H.; Sivaraj, K.K.; Hoxha, E.; Cuesta-Domínguez, Á.; Pinho, S.; Akhmetzyanova, I.; Gao, J.; Witkowski, M.; et al. The Bone Marrow Microenvironment at Single-Cell Resolution. Nature 2019, 569, 222–228. [Google Scholar] [CrossRef]
- Zhong, L.; Yao, L.; Tower, R.J.; Wei, Y.; Miao, Z.; Park, J.; Shrestha, R.; Wang, L.; Yu, W.; Holdreith, N.; et al. Single Cell Transcriptomics Identifies a Unique Adipose Lineage Cell Population That Regulates Bone Marrow Environment. eLife 2020, 9, e54695. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Zhong, L.; Yao, L.; Wei, Y.; Gui, T.; Li, Z.; Kim, H.; Holdreith, N.; Jiang, X.; Tong, W.; et al. Bone Marrow Adipogenic Lineage Precursors Promote Osteoclastogenesis in Bone Remodeling and Pathologic Bone Loss. J. Clin. Investig. 2021, 131, e140214. [Google Scholar] [CrossRef]
- Onji, M.; Werschler, N.; Penninger, J. A Critical Relationship between Bone and Fat: The Role of Bone Marrow Adipose-Derived RANKL in Bone Metabolism. EMBO Rep. 2021, 22, e52986. [Google Scholar] [CrossRef]
- Reagan, M.R. Critical Assessment of In Vitro and In Vivo Models to Study Marrow Adipose Tissue. Curr. Osteoporos. Rep. 2020, 18, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Aaron, N.; Kraakman, M.J.; Zhou, Q.; Liu, Q.; Costa, S.; Yang, J.; Liu, L.; Yu, L.; Wang, L.; He, Y.; et al. Adipsin Promotes Bone Marrow Adiposity by Priming Mesenchymal Stem Cells. eLife 2021, 10, e69209. [Google Scholar] [CrossRef] [PubMed]
- Aaron, N.; Costa, S.; Rosen, C.J.; Qiang, L. The Implications of Bone Marrow Adipose Tissue on Inflammaging. Front. Endocrinol. 2022, 13, 853765. [Google Scholar] [CrossRef] [PubMed]
- Shay, A.E.; Diwakar, B.T.; Guan, B.-J.; Narayan, V.; Urban, J.F.; Prabhu, K.S. IL-4 up-Regulates Cyclooxygenase-1 Expression in Macrophages. J. Biol. Chem. 2017, 292, 14544–14555. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.-F.; Shen, W.-J.; Ueno, M.; Patel, S.; Kraemer, F.B. Characterization of Age-Related Gene Expression Profiling in Bone Marrow and Epididymal Adipocytes. BMC Genom. 2011, 12, 212. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Hanai, J.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017, 25, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Scheller, E.L.; Khandaker, S.; Learman, B.S.; Cawthorn, W.P.; Anderson, L.M.; Pham, H.A.; Robles, H.; Wang, Z.; Li, Z.; Parlee, S.D.; et al. Bone Marrow Adipocytes Resist Lipolysis and Remodeling in Response to β-Adrenergic Stimulation. Bone 2019, 118, 32–41. [Google Scholar] [CrossRef]
- Suchacki, K.J.; Tavares, A.A.S.; Mattiucci, D.; Scheller, E.L.; Papanastasiou, G.; Gray, C.; Sinton, M.C.; Ramage, L.E.; McDougald, W.A.; Lovdel, A.; et al. Bone Marrow Adipose Tissue Is a Unique Adipose Subtype with Distinct Roles in Glucose Homeostasis. Nat. Commun. 2020, 11, 3097. [Google Scholar] [CrossRef]
- Pham, T.T.; Ivaska, K.K.; Hannukainen, J.C.; Virtanen, K.A.; Lidell, M.E.; Enerbäck, S.; Mäkelä, K.; Parkkola, R.; Piirola, S.; Oikonen, V.; et al. Human Bone Marrow Adipose Tissue Is a Metabolically Active and Insulin-Sensitive Distinct Fat Depot. J. Clin. Endocrinol. Metab. 2020, 105, 2300–2310. [Google Scholar] [CrossRef]
- Labella, R.; Vujačić, M.; Trivanović, D. Bone Marrow Adipose Tissue: Regulation of Osteoblastic Niche, Hematopoiesis and Hematological Malignancies. Stem Cell Rev. Rep. 2023, 19, 1135–1151. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, G.; Shehu, D.; Galli, C. Factors Controlling Haemopoiesis in Ovine Long Term Bone Marrow Cultures. Vet. Immunol. Immunopathol. 1997, 55, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Naveiras, O.; Nardi, V.; Wenzel, P.L.; Hauschka, P.V.; Fahey, F.; Daley, G.Q. Bone-Marrow Adipocytes as Negative Regulators of the Haematopoietic Microenvironment. Nature 2009, 460, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.-M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schürmann, A.; et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 2017, 20, 771–784.e6. [Google Scholar] [CrossRef] [Green Version]
- Infante, A.; Rodríguez, C.I. Osteogenesis and Aging: Lessons from Mesenchymal Stem Cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Josephson, A.M.; Bradaschia-Correa, V.; Lee, S.; Leclerc, K.; Patel, K.S.; Muinos Lopez, E.; Litwa, H.P.; Neibart, S.S.; Kadiyala, M.; Wong, M.Z.; et al. Age-Related Inflammation Triggers Skeletal Stem/Progenitor Cell Dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 6995–7004. [Google Scholar] [CrossRef] [Green Version]
- Miggitsch, C.; Meryk, A.; Naismith, E.; Pangrazzi, L.; Ejaz, A.; Jenewein, B.; Wagner, S.; Nägele, F.; Fenkart, G.; Trieb, K.; et al. Human Bone Marrow Adipocytes Display Distinct Immune Regulatory Properties. EBioMedicine 2019, 46, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Schuh, J.C.L. Hepatic Nodular Myelolipomatosis (Myelolipomas) Associated with a Peritoneo-Pericardial Diaphragmatic Hernia in a Cat. J. Comp. Pathol. 1987, 97, 231–235. [Google Scholar] [CrossRef]
- Calissendorff, J.; Juhlin, C.C.; Sundin, A.; Bancos, I.; Falhammar, H. Adrenal Myelolipomas. Lancet Diabetes Endocrinol. 2021, 9, 767–775. [Google Scholar] [CrossRef]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D.; Mizoguchi, T.; Wei, Q.; Lucas, D.; Ito, K.; et al. Arteriolar Niches Maintain Haematopoietic Stem Cell Quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Robino, J.J.; Pamir, N.; Rosario, S.; Crawford, L.B.; Burwitz, B.J.; Roberts, C.T.; Kurre, P.; Varlamov, O. Spatial and Biochemical Interactions between Bone Marrow Adipose Tissue and Hematopoietic Stem and Progenitor Cells in Rhesus Macaques. Bone 2020, 133, 115248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.O.; Yu, H.; Yue, R.; Zhao, Z.; Rios, J.J.; Naveiras, O.; Morrison, S.J. Bone Marrow Adipocytes Promote the Regeneration of Stem Cells and Haematopoiesis by Secreting SCF. Nat. Cell Biol. 2017, 19, 891–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Huang, Z.; Ong, B.; Sahu, C.; Zeng, H.; Ruan, H.-B. Bone Marrow Adipose Tissue-Derived Stem Cell Factor Mediates Metabolic Regulation of Hematopoiesis. Haematologica 2019, 104, 1731–1743. [Google Scholar] [CrossRef] [Green Version]
- Mostoufi-Moab, S.; Magland, J.; Isaacoff, E.J.; Sun, W.; Rajapakse, C.S.; Zemel, B.; Wehrli, F.; Shekdar, K.; Baker, J.; Long, J.; et al. Adverse Fat Depots and Marrow Adiposity Are Associated With Skeletal Deficits and Insulin Resistance in Long-Term Survivors of Pediatric Hematopoietic Stem Cell Transplantation. J. Bone Miner. Res. 2015, 30, 1657–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, D.; Gaddy, D.; Suva, L.J.; Corry, P.M. Rapid Loss of Bone Mass and Strength in Mice after Abdominal Irradiation. Radiat. Res. 2011, 176, 624–635. [Google Scholar] [CrossRef] [Green Version]
- Fazeli, P.K.; Bredella, M.A.; Pachon-Peña, G.; Zhao, W.; Zhang, X.; Faje, A.T.; Resulaj, M.; Polineni, S.P.; Holmes, T.M.; Lee, H.; et al. The Dynamics of Human Bone Marrow Adipose Tissue in Response to Feeding and Fasting. JCI Insight 2021, 6, e138636. [Google Scholar] [CrossRef]
- Veldhuis-Vlug, A.G.; Rosen, C.J. Clinical Implications of Bone Marrow Adiposity. J. Intern. Med. 2018, 283, 121–139. [Google Scholar] [CrossRef] [Green Version]
- Doucette, C.R.; Horowitz, M.C.; Berry, R.; MacDougald, O.A.; Anunciado-Koza, R.; Koza, R.A.; Rosen, C.J. A High Fat Diet Increases Bone Marrow Adipose Tissue (MAT) But Does Not Alter Trabecular or Cortical Bone Mass in C57BL/6J Mice. J. Cell. Physiol. 2015, 230, 2032–2037. [Google Scholar] [CrossRef] [Green Version]
- Corre, J.; Planat-Benard, V.; Corberand, J.X.; Pénicaud, L.; Casteilla, L.; Laharrague, P. Human Bone Marrow Adipocytes Support Complete Myeloid and Lymphoid Differentiation from Human CD34+ Cells. Br. J. Haematol. 2004, 127, 344–347. [Google Scholar] [CrossRef]
- Gimble, J.M.; Nuttall, M.E. The Relationship between Adipose Tissue and Bone Metabolism. Clin. Biochem. 2012, 45, 874–879. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Meryk, A.; Naismith, E.; Koziel, R.; Lair, J.; Krismer, M.; Trieb, K.; Grubeck-Loebenstein, B. “Inflamm-Aging” Influences Immune Cell Survival Factors in Human Bone Marrow. Eur. J. Immunol. 2017, 47, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Kwak, H.-J.; Liu, P.; Bajrami, B.; Xu, Y.; Park, S.-Y.; Nombela-Arrieta, C.; Mondal, S.; Kambara, H.; Yu, H.; et al. Reactive Oxygen Species–Producing Myeloid Cells Act as a Bone Marrow Niche for Sterile Inflammation–Induced Reactive Granulopoiesis. J. Immunol. 2017, 198, 2854–2864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, T.; Ho, J.H.; Yang, M.-H.; Lee, O.K. Glucose Reduction Prevents Replicative Senescence and Increases Mitochondrial Respiration in Human Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-C.; Hsu, M.-F.; Wu, K.K. High Glucose Induces Bone Marrow-Derived Mesenchymal Stem Cell Senescence by Upregulating Autophagy. PLoS ONE 2015, 10, e0126537. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.H.; Lee, J.H.; Lee, K.M.; Lee, C.-K.; Shin, D.-M. BMP-2 Induced Signaling Pathways and Phenotypes: Comparisons Between Senescent and Non-Senescent Bone Marrow Mesenchymal Stem Cells. Calcif. Tissue Int. 2022, 110, 489–503. [Google Scholar] [CrossRef]
- Li, H.; Liu, P.; Xu, S.; Li, Y.; Dekker, J.D.; Li, B.; Fan, Y.; Zhang, Z.; Hong, Y.; Yang, G.; et al. FOXP1 Controls Mesenchymal Stem Cell Commitment and Senescence during Skeletal Aging. J. Clin. Investig. 2017, 127, 1241–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, R.; Banerjee, I.; Manna, D.; Reghupaty, S.C.; Yetirajam, R.; Sarkar, D. Mouse Bone Marrow Cell Isolation and Macrophage Differentiation. Methods Mol. Biol. 2022, 2455, 85–91. [Google Scholar] [CrossRef]
- Inoue, K.; Qin, Y.; Xia, Y.; Han, J.; Yuan, R.; Sun, J.; Xu, R.; Jiang, J.X.; Greenblatt, M.B.; Zhao, B. Bone Marrow Adipoq-Lineage Progenitors Are a Major Cellular Source of M-CSF That Dominates Bone Marrow Macrophage Development, Osteoclastogenesis, and Bone Mass. eLife 2023, 12, e82118. [Google Scholar] [CrossRef] [PubMed]
- Nour, J.; Moregola, A.; Svecla, M.; Da Dalt, L.; Bellini, R.; Neyrolles, O.; Fadini, G.P.; Rombouts, Y.; Albiero, M.; Bonacina, F.; et al. Mannose Receptor Deficiency Impacts Bone Marrow and Circulating Immune Cells during High Fat Diet Induced Obesity. Metabolites 2022, 12, 1205. [Google Scholar] [CrossRef]
- Xu, Y.; Murphy, A.J.; Fleetwood, A.J. Hematopoietic Progenitors and the Bone Marrow Niche Shape the Inflammatory Response and Contribute to Chronic Disease. Int. J. Mol. Sci. 2022, 23, 2234. [Google Scholar] [CrossRef]
- Varghese, M.; Griffin, C.; Abrishami, S.; Eter, L.; Lanzetta, N.; Hak, L.; Clemente, J.; Agarwal, D.; Lerner, A.; Westerhoff, M.; et al. Sex Hormones Regulate Metainflammation in Diet-Induced Obesity in Mice. J. Biol. Chem. 2021, 297, 101229. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Clemente, J.; Lerner, A.; Abrishami, S.; Islam, M.; Subbaiah, P.; Singer, K. Monocyte Trafficking and Polarization Contribute to Sex Differences in Meta-Inflammation. Front. Endocrinol. 2022, 13, 826320. [Google Scholar] [CrossRef] [PubMed]
- Potteaux, S.; Ait-Oufella, H.; Mallat, Z. Role of Splenic Monocytes in Atherosclerosis. Curr. Opin. Lipidol. 2015, 26, 457–463. [Google Scholar] [CrossRef]
- Liu, S.; Szatmary, P.; Lin, J.-W.; Wang, Q.; Sutton, R.; Chen, L.; Liu, T.; Huang, W.; Xia, Q. Circulating Monocytes in Acute Pancreatitis. Front. Immunol. 2022, 13, 1062849. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Günther, P.; Cirovic, B.; Baßler, K.; Händler, K.; Becker, M.; Dutertre, C.A.; Bigley, V.; Newell, E.; Collin, M.; Ginhoux, F.; et al. A Rule-Based Data-Informed Cellular Consensus Map of the Human Mononuclear Phagocyte Cell Space. bioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Tak, T.; van Groenendael, R.; Pickkers, P.; Koenderman, L. Monocyte Subsets Are Differentially Lost from the Circulation during Acute Inflammation Induced by Human Experimental Endotoxemia. J. Innate Immun. 2017, 9, 464–474. [Google Scholar] [CrossRef]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.P.; Thomas, G.D.; Hedrick, C.C. 2014 Jeffrey M. Hoeg Award Lecture. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1722–1733. [Google Scholar] [CrossRef] [Green Version]
- Hofer, T.P.; Zawada, A.M.; Frankenberger, M.; Skokann, K.; Satzl, A.A.; Gesierich, W.; Schuberth, M.; Levin, J.; Danek, A.; Rotter, B.; et al. Slan-Defined Subsets of CD16-Positive Monocytes: Impact of Granulomatous Inflammation and M-CSF Receptor Mutation. Blood 2015, 126, 2601–2610. [Google Scholar] [CrossRef] [Green Version]
- Zawada, A.M.; Fell, L.H.; Untersteller, K.; Seiler, S.; Rogacev, K.S.; Fliser, D.; Ziegler-Heitbrock, L.; Heine, G.H. Comparison of Two Different Strategies for Human Monocyte Subsets Gating within the Large-Scale Prospective CARE FOR HOMe Study. Cytom. A 2015, 87, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Waller, K.; James, C.; de Jong, A.; Blackmore, L.; Ma, Y.; Stagg, A.; Kelsell, D.; O’Dwyer, M.; Hutchins, R.; Alazawi, W. ADAM17-Mediated Reduction in CD14++CD16+ Monocytes Ex Vivo and Reduction in Intermediate Monocytes With Immune Paresis in Acute Pancreatitis and Acute Alcoholic Hepatitis. Front. Immunol. 2019, 10, 1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.A.; Zhang, Y.; Fullerton, J.N.; Boelen, L.; Rongvaux, A.; Maini, A.A.; Bigley, V.; Flavell, R.A.; Gilroy, D.W.; Asquith, B.; et al. The Fate and Lifespan of Human Monocyte Subsets in Steady State and Systemic Inflammation. J. Exp. Med. 2017, 214, 1913–1923. [Google Scholar] [CrossRef]
- Tak, T.; Drylewicz, J.; Conemans, L.; de Boer, R.J.; Koenderman, L.; Borghans, J.A.M.; Tesselaar, K. Circulatory and Maturation Kinetics of Human Monocyte Subsets in Vivo. Blood 2017, 130, 1474–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, X.; Zhong, J.; Sun, Q. The Heterogenic Properties of Monocytes/Macrophages and Neutrophils in Inflammatory Response in Diabetes. Life Sci. 2014, 116, 59–66. [Google Scholar] [CrossRef]
- Devesa, A.; Lobo-González, M.; Martínez-Milla, J.; Oliva, B.; García-Lunar, I.; Mastrangelo, A.; España, S.; Sanz, J.; Mendiguren, J.M.; Bueno, H.; et al. Bone Marrow Activation in Response to Metabolic Syndrome and Early Atherosclerosis. Eur. Heart J. 2022, 43, 1809–1828. [Google Scholar] [CrossRef]
- Hoyer, F.F.; Zhang, X.; Coppin, E.; Vasamsetti, S.B.; Modugu, G.; Schloss, M.J.; Rohde, D.; McAlpine, C.S.; Iwamoto, Y.; Libby, P.; et al. Bone Marrow Endothelial Cells Regulate Myelopoiesis in Diabetes Mellitus. Circulation 2020, 142, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Wijngaarden, L.H.; van der Harst, E.; Klaassen, R.A.; Dunkelgrun, M.; Kuijper, T.M.; Klepper, M.; Ambagtsheer, G.; IJzermans, J.N.M.; de Bruin, R.W.F.; Litjens, N.H.R. Effects of Morbid Obesity and Metabolic Syndrome on the Composition of Circulating Immune Subsets. Front. Immunol. 2021, 12, 675018. [Google Scholar] [CrossRef] [PubMed]
- Poitou, C.; Dalmas, E.; Renovato, M.; Benhamo, V.; Hajduch, F.; Abdennour, M.; Kahn, J.-F.; Veyrie, N.; Rizkalla, S.; Fridman, W.-H.; et al. CD14dimCD16+ and CD14+CD16+ Monocytes in Obesity and during Weight Loss: Relationships with Fat Mass and Subclinical Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2322–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grün, J.L.; Manjarrez-Reyna, A.N.; Gómez-Arauz, A.Y.; Leon-Cabrera, S.; Rückert, F.; Fragoso, J.M.; Bueno-Hernández, N.; Islas-Andrade, S.; Meléndez-Mier, G.; Escobedo, G. High-Density Lipoprotein Reduction Differentially Modulates to Classical and Nonclassical Monocyte Subpopulations in Metabolic Syndrome Patients and in LPS-Stimulated Primary Human Monocytes In Vitro. J. Immunol. Res. 2018, 2018, e2737040. [Google Scholar] [CrossRef] [Green Version]
- Russo, L.; Lumeng, C.N. Properties and Functions of Adipose Tissue Macrophages in Obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, S.; Tung, N.; Casanova-Acebes, M.; Chang, C.; Cantoni, C.; Zhang, D.; Wirtz, T.H.; Naik, S.; Rose, S.A.; Brocker, C.N.; et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell 2019, 178, 1102–1114.e17. [Google Scholar] [CrossRef] [PubMed]
- Devêvre, E.F.; Renovato-Martins, M.; Clément, K.; Sautès-Fridman, C.; Cremer, I.; Poitou, C. Profiling of the Three Circulating Monocyte Subpopulations in Human Obesity. J. Immunol. 2015, 194, 3917–3923. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, K.; Sommer, M.; Strobel, S.; Thrum, S.; Blüher, M.; Wagner, U.; Rossol, M. Perturbation of the Monocyte Compartment in Human Obesity. Front. Immunol. 2019, 10, 1874. [Google Scholar] [CrossRef] [Green Version]
- Pecht, T.; Haim, Y.; Bashan, N.; Shapiro, H.; Harman-Boehm, I.; Kirshtein, B.; Clément, K.; Shai, I.; Rudich, A. Circulating Blood Monocyte Subclasses and Lipid-Laden Adipose Tissue Macrophages in Human Obesity. PLoS ONE 2016, 11, e0159350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Lin, G.; Zhou, J.; Mund, J.A.; Case, J.; Campbell, W.W. Weight Loss Achieved Using an Energy Restriction Diet with Normal or Higher Dietary Protein Decreased the Number of CD14++CD16+ Proinflammatory Monocytes and Plasma Lipids and Lipoproteins in Middle-Aged, Overweight, and Obese Adults. Nutr. Res. 2017, 40, 75–84. [Google Scholar] [CrossRef] [Green Version]
- de Matos, M.A.; Duarte, T.C.; Ottone, V.D.O.; da Sampaio, M.P.F.; Costa, K.B.; de Oliveira, M.F.A.; Moseley, P.L.; Schneider, S.M.; Coimbra, C.C.; Brito-Melo, G.E.A.; et al. The Effect of Insulin Resistance and Exercise on the Percentage of CD16+ Monocyte Subset in Obese Individuals. Cell Biochem. Funct. 2016, 34, 209–216. [Google Scholar] [CrossRef]
- van der Valk, E.S.; Mulder, D.S.; Kouwenhoven, T.; Nagtzaam, N.M.A.; van Rossum, E.F.C.; Dik, W.A.; Leenen, P.J.M. Monocyte Adaptations in Patients with Obesity during a 1.5 Year Lifestyle Intervention. Front. Immunol. 2022, 13, 1022361. [Google Scholar] [CrossRef]
- van der Zalm, I.J.B.; van der Valk, E.S.; Wester, V.L.; Nagtzaam, N.M.A.; van Rossum, E.F.C.; Leenen, P.J.M.; Dik, W.A. Obesity-Associated T-Cell and Macrophage Activation Improve Partly after a Lifestyle Intervention. Int. J. Obes. 2020, 44, 1838–1850. [Google Scholar] [CrossRef]
- Connaughton, E.P.; Naicker, S.; Hanley, S.A.; Slevin, S.M.; Eykelenboom, J.K.; Lowndes, N.F.; O’Brien, T.; Ceredig, R.; Griffin, M.D.; Dennedy, M.C. Phenotypic and Functional Heterogeneity of Human Intermediate Monocytes Based on HLA-DR Expression. Immunol. Cell Biol. 2018, 96, 742–758. [Google Scholar] [CrossRef] [Green Version]
- Hildreth, A.D.; Ma, F.; Wong, Y.Y.; Sun, R.; Pellegrini, M.; O’Sullivan, T.E. Single-Cell Sequencing of Human White Adipose Tissue Identifies New Cell States in Health and Obesity. Nat. Immunol. 2021, 22, 639–653. [Google Scholar] [CrossRef]
- Daemen, S.; Schilling, J.D. The Interplay Between Tissue Niche and Macrophage Cellular Metabolism in Obesity. Front. Immunol. 2019, 10, 3133. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Ni, L.; Zhuge, F.; Xu, L.; Fu, Z.; Ota, T. Adipose Tissue Macrophage Phenotypes and Characteristics: The Key to Insulin Resistance in Obesity and Metabolic Disorders. Obesity 2020, 28, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Guria, S.; Hoory, A.; Das, S.; Chattopadhyay, D.; Mukherjee, S. Adipose Tissue Macrophages and Their Role in Obesity-Associated Insulin Resistance: An Overview of the Complex Dynamics at Play. Biosci. Rep. 2023, 43, BSR20220200. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Lim, H.-W.; Kim, Y.H.; Ho, W.Y.; Foong, Y.H.; Nelson, V.L.; Nguyen, H.C.B.; Chegireddy, K.; Kim, J.; Habertheuer, A.; et al. Distinct Macrophage Populations Direct Inflammatory versus Physiological Changes in Adipose Tissue. Proc. Natl. Acad. Sci. USA 2018, 115, E5096–E5105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaitin, D.A.; Adlung, L.; Thaiss, C.A.; Weiner, A.; Li, B.; Descamps, H.; Lundgren, P.; Bleriot, C.; Liu, Z.; Deczkowska, A.; et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 2019, 178, 686–698.e14. [Google Scholar] [CrossRef]
- Pirzgalska, R.M.; Seixas, E.; Seidman, J.S.; Link, V.M.; Sánchez, N.M.; Mahú, I.; Mendes, R.; Gres, V.; Kubasova, N.; Morris, I.; et al. Sympathetic Neuron–Associated Macrophages Contribute to Obesity by Importing and Metabolizing Norepinephrine. Nat. Med. 2017, 23, 1309–1318. [Google Scholar] [CrossRef]
- Stansbury, C.M.; Dotson, G.A.; Pugh, H.; Rehemtulla, A.; Rajapakse, I.; Muir, L.A. A Lipid-Associated Macrophage Lineage Rewires the Spatial Landscape of Adipose Tissue in Early Obesity. bioRxiv 2023. [Google Scholar] [CrossRef]
- Jais, A.; Brüning, J.C. Hypothalamic Inflammation in Obesity and Metabolic Disease. J. Clin. Investig. 2017, 127, 24–32. [Google Scholar] [CrossRef]
- Kälin, S.; Heppner, F.L.; Bechmann, I.; Prinz, M.; Tschöp, M.H.; Yi, C.-X. Hypothalamic Innate Immune Reaction in Obesity. Nat. Rev. Endocrinol. 2015, 11, 339–351. [Google Scholar] [CrossRef]
- Lee, C.H.; Shin, S.H.; Kang, G.M.; Kim, S.; Kim, J.; Yu, R.; Kim, M.-S. Cellular Source of Hypothalamic Macrophage Accumulation in Diet-Induced Obesity. J. Neuroinflamm. 2019, 16, 221. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metab. 2017, 26, 185–197.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morari, J.; Anhe, G.F.; Nascimento, L.F.; de Moura, R.F.; Razolli, D.; Solon, C.; Guadagnini, D.; Souza, G.; Mattos, A.H.; Tobar, N.; et al. Fractalkine (CX3CL1) Is Involved in the Early Activation of Hypothalamic Inflammation in Experimental Obesity. Diabetes 2014, 63, 3770–3784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, V.L.; He, Y.; Contrepois, K.; Liu, H.; Kim, J.T.; Wiggenhorn, A.L.; Tanzo, J.T.; Tung, A.S.-H.; Lyu, X.; Zushin, P.-J.H.; et al. An Exercise-Inducible Metabolite That Suppresses Feeding and Obesity. Nature 2022, 606, 785–790. [Google Scholar] [CrossRef]
- Wadley, A.J.; Roberts, M.J.; Creighton, J.; Thackray, A.E.; Stensel, D.J.; Bishop, N.C. Higher Levels of Physical Activity Are Associated with Reduced Tethering and Migration of Pro-Inflammatory Monocytes in Males with Central Obesity. Exerc. Immunol. Rev. 2021, 27, 54–66. [Google Scholar]
- Emmons, R.; Niemiro, G.M.; De Lisio, M. Hematopoiesis with Obesity and Exercise: Role of the Bone Marrow Niche. Exerc. Immunol. Rev. 2017, 23, 82–95. [Google Scholar]
- Breznik, J.A.; Naidoo, A.; Foley, K.P.; Schulz, C.; Lau, T.C.; Loukov, D.; Sloboda, D.M.; Bowdish, D.M.E.; Schertzer, J.D. TNF, but Not Hyperinsulinemia or Hyperglycemia, Is a Key Driver of Obesity-Induced Monocytosis Revealing That Inflammatory Monocytes Correlate with Insulin in Obese Male Mice. Physiol. Rep. 2018, 6, e13937. [Google Scholar] [CrossRef] [Green Version]
- Phu, T.A.; Ng, M.; Vu, N.K.; Bouchareychas, L.; Raffai, R.L. IL-4 Polarized Human Macrophage Exosomes Control Cardiometabolic Inflammation and Diabetes in Obesity. Mol. Ther. 2022, 30, 2274–2297. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todosenko, N.; Khaziakhmatova, O.; Malashchenko, V.; Yurova, K.; Bograya, M.; Beletskaya, M.; Vulf, M.; Mikhailova, L.; Minchenko, A.; Soroko, I.; et al. Adipocyte- and Monocyte-Mediated Vicious Circle of Inflammation and Obesity (Review of Cellular and Molecular Mechanisms). Int. J. Mol. Sci. 2023, 24, 12259. https://doi.org/10.3390/ijms241512259
Todosenko N, Khaziakhmatova O, Malashchenko V, Yurova K, Bograya M, Beletskaya M, Vulf M, Mikhailova L, Minchenko A, Soroko I, et al. Adipocyte- and Monocyte-Mediated Vicious Circle of Inflammation and Obesity (Review of Cellular and Molecular Mechanisms). International Journal of Molecular Sciences. 2023; 24(15):12259. https://doi.org/10.3390/ijms241512259
Chicago/Turabian StyleTodosenko, Natalia, Olga Khaziakhmatova, Vladimir Malashchenko, Kristina Yurova, Maria Bograya, Maria Beletskaya, Maria Vulf, Larisa Mikhailova, Anastasia Minchenko, Irina Soroko, and et al. 2023. "Adipocyte- and Monocyte-Mediated Vicious Circle of Inflammation and Obesity (Review of Cellular and Molecular Mechanisms)" International Journal of Molecular Sciences 24, no. 15: 12259. https://doi.org/10.3390/ijms241512259
APA StyleTodosenko, N., Khaziakhmatova, O., Malashchenko, V., Yurova, K., Bograya, M., Beletskaya, M., Vulf, M., Mikhailova, L., Minchenko, A., Soroko, I., Khlusov, I., & Litvinova, L. (2023). Adipocyte- and Monocyte-Mediated Vicious Circle of Inflammation and Obesity (Review of Cellular and Molecular Mechanisms). International Journal of Molecular Sciences, 24(15), 12259. https://doi.org/10.3390/ijms241512259