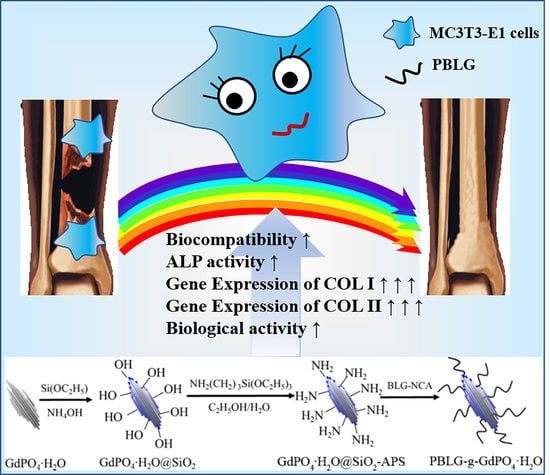
Surface Biofunctionalization of Gadolinium Phosphate Nanobunches for Boosting Osteogenesis/Chondrogenesis Differentiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design and Synthesis of PBLG-g-GdPO4·H2O
2.1.1. Sufficient Suitable Encapsulation of SiO2 onto the Surface of GdPO4·H2O Nanobunches
2.1.2. Further Surface Modification of GdPO4·H2O@SiO2 with Polymer APS and PBLG
2.2. Dispersion and Stability
2.3. T1-Weighted MR Imaging of Modifiers in Biomedical Polymer Material of PLGA
2.4. Cellular Response
2.4.1. Cytotoxicity and Biocompatibility
2.4.2. Osteogenic/Chondrogenic Induction Activity
2.5. Possible Molecular Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of GdPO4·H2O@SiO2
3.3. Synthesis of GdPO4·H2O@SiO2–APS and BLG-NCA
3.4. Synthesis of PBLG-g-GdPO4·H2O
3.5. Preparation of Nanocomposites for MR Imaging and Cell Experiments
3.6. Characterization of Physical and Chemical Properties
3.7. Magnetic Resonance Imaging In Vitro
3.8. In Vitro Cell Culture
3.9. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, M.; Shaitan, K.; Qu, X.; Bonartsev, A.P.; Lei, B. Bioactive rare earth-based inorganic-organic hybrid biomaterials for wound healing and repair. Appl. Mater. Today 2022, 26, 101304. [Google Scholar] [CrossRef]
- Pozdniakova, N.V.; Ryabaya, O.V.; Semkina, A.S.; Skribitsky, V.A.; Shevelev, A.B. Using ELP Repeats as a Scaffold for De Novo Construction of Gadolinium-Binding Domains within Multifunctional Recombinant Proteins for Targeted Delivery of Gadolinium to Tumour Cells. Int. J. Mol. Sci. 2022, 23, 3297. [Google Scholar] [CrossRef]
- Shapoval, O.; Sulimenko, V.; Klebanovych, A.; Rabyk, M.; Shapoval, P.; Kaman, O.; Rydvalová, E.; Filipová, M.; Dráberová, E.; Dráber, P.; et al. Multimodal fluorescently labeled polymer-coated GdF3 nanoparticles inhibit degranulation in mast cells. Nanoscale 2021, 13, 19023–19037. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Shi, S.; Wu, Z.; Huang, Y.; Ji, C.; Grimes, C.A.; Feng, X.; Cai, Q. Lanthanide/Cu2–xSe Nanoparticles for Bacteria-Activated NIR-II Fluorescence Imaging of Infection. ACS Sens. 2022, 7, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Hsieh, H.-H.; Chang, T.-Y.; Lin, J.-J.; Wu, C.-C.; Hsu, M.-H.; Lin, M.-C.; Peng, S.-L. Development of MRI-Detectable Boron-Containing Gold Nanoparticle-Encapsulated Biodegradable Polymeric Matrix for Boron Neutron Capture Therapy (BNCT). Int. J. Mol. Sci. 2021, 22, 8050. [Google Scholar] [CrossRef]
- Corey, Z.J.; Lu, P.; Zhang, G.; Sharma, Y.; Rutherford, B.X.; Dhole, S.; Roy, P.; Wang, Z.; Wu, Y.; Wang, H.; et al. Structural and Optical Properties of High Entropy (La,Lu,Y,Gd,Ce)AlO3 Perovskite Thin Films. Adv. Sci. 2022, 9, 2202671. [Google Scholar] [CrossRef]
- Pu, R.; Zhan, Q.; Peng, X.; Liu, S.; Guo, X.; Liang, L.; Qin, X.; Zhao, Z.W.; Liu, X. Super-resolution microscopy enabled by high-efficiency surface-migration emission depletion. Nat. Commun. 2022, 13, 6636. [Google Scholar] [CrossRef]
- Natarajan, D.; Ye, Z.; Wang, L.; Ge, L.; Pathak, J.L. Rare earth smart nanomaterials for bone tissue engineering and implantology: Advances, challenges, and prospects. Bioeng. Transl. Med. 2022, 7, e10262. [Google Scholar] [CrossRef]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Bozorgi, A.; Khazaei, M.; Soleimani, M.; Jamalpoor, Z. Application of nanoparticles in bone tissue engineering; a review on the molecular mechanisms driving osteogenesis. Biomater. Sci. 2021, 9, 4541–4567. [Google Scholar] [CrossRef]
- Nethi, S.K.; Veeriah, V.; Barui, A.K.; Rajendran, S.; Mattapally, S.; Misra, S.; Chatterjee, S.; Patra, C.R. Investigation of molecular mechanisms and regulatory pathways of pro-angiogenic nanorods. Nanoscale 2015, 7, 9760–9770. [Google Scholar] [CrossRef] [Green Version]
- Patra, C.R.; Bhattacharya, R.; Patra, S.; Vlahakis, N.E.; Gabashvili, A.; Koltypin, Y.; Gedanken, A.; Mukherjee, P.; Mukhopadhyay, D. Pro-angiogenic Properties of Europium(III) Hydroxide Nanorods. Adv. Mater. 2008, 20, 753–756. [Google Scholar] [CrossRef]
- Li, J.; Kang, F.; Gong, X.; Bai, Y.; Dai, J.; Zhao, C.; Dou, C.; Cao, Z.; Liang, M.; Dong, R.; et al. Ceria nanoparticles enhance endochondral ossification–based critical-sized bone defect regeneration by promoting the hypertrophic differentiation of BMSCs via DHX15 activation. FASEB J. 2019, 33, 6378–6389. [Google Scholar] [CrossRef]
- Liao, F.; Peng, X.-Y.; Yang, F.; Ke, Q.-F.; Zhu, Z.-H.; Guo, Y.-P. Gadolinium-doped mesoporous calcium silicate/chitosan scaffolds enhanced bone regeneration ability. Mater. Sci. Eng. C 2019, 104, 109999. [Google Scholar] [CrossRef]
- Zhu, D.-Y.; Lu, B.; Yin, J.-H.; Ke, Q.-F.; Xu, H.; Zhang, C.-Q.; Guo, Y.-P.; Gao, Y.-S. Gadolinium-doped bioglass scaffolds promote osteogenic differentiation of hBMSC via the Akt/GSK3β pathway and facilitate bone repair in vivo. Int. J. Nanomed. 2019, 14, 1085. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Ni, N.; Su, Y.; Miao, H.; Tang, Z.; Ji, Y.; Wang, Y.; Gao, H.; Ju, Y.; Sun, N.; et al. Targeting Local Osteogenic and Ancillary Cells by Mechanobiologically Optimized Magnesium Scaffolds for Orbital Bone Reconstruction in Canines. ACS Appl. Mater. Interfaces 2020, 12, 27889–27904. [Google Scholar] [CrossRef]
- Mastrogiacomo, S.; Kownacka, A.E.; Dou, W.; Burke, B.P.; de Rosales, R.T.M.; Heerschap, A.; Jansen, J.A.; Archibald, S.J.; Walboomers, X.F. Bisphosphonate Functionalized Gadolinium Oxide Nanoparticles Allow Long-Term MRI/CT Multimodal Imaging of Calcium Phosphate Bone Cement. Adv. Healthc. Mater. 2018, 7, 1800202. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Lv, Z.; Wang, Y.; Wang, Z.; Gao, T.; Zhang, N.; Guo, M.; Zou, H.; Zhang, P. In vivo MRI and X-Ray Bifunctional Imaging of Polymeric Composite Supplemented with GdPO4·H2O Nanobundles for Tracing Bone Implant and Bone Regeneration. Adv. Healthc. Mater. 2016, 5, 2182–2190. [Google Scholar] [CrossRef]
- Zhao, P.-P.; Hu, H.-R.; Liu, J.-Y.; Ke, Q.-F.; Peng, X.-Y.; Ding, H.; Guo, Y.-P. Gadolinium phosphate/chitosan scaffolds promote new bone regeneration via Smad/Runx2 pathway. Chem. Eng. J. 2019, 359, 1120–1129. [Google Scholar] [CrossRef]
- Samadian, S.; Karbalaei, A.; Pourmadadi, M.; Yazdian, F.; Malmir, S.J. A novel alginate-gelatin microcapsule to enhance bone differentiation of mesenchymal stem cells. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 395–402. [Google Scholar] [CrossRef]
- Yu, C.; Li, K.; Xu, L.; Li, B.; Li, C.; Guo, S.; Li, Z.; Zhang, Y.; Hussain, A.; Tan, H.; et al. siRNA-functionalized lanthanide nanoparticle enables efficient endosomal escape and cancer treatment. Nano Res. 2022, 15, 9160–9168. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, X.; Peng, J.; Li, F. Core–Shell Lanthanide Upconversion Nanophosphors as Four-Modal Probes for Tumor Angiogenesis Imaging. ACS Nano 2013, 7, 11290–11300. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zeng, X.; Chen, H.; Li, Z.; Zeng, W.; Mei, L.; Zhao, Y. Versatile Polydopamine Platforms: Synthesis and Promising Applications for Surface Modification and Advanced Nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, C.; Li, D.; Zhao, L. Rational surface modification of gadolinium borate nanoparticles enhancing colloidal stability in physiological media for potential neutron capture therapy and magnetic resonance imaging. Colloids Surf. B. Biointerfaces 2022, 218, 112771. [Google Scholar] [CrossRef]
- Pichaandi, J.; Abel, K.A.; Johnson, N.J.J.; van Veggel, F.C.J.M. Long-Term Colloidal Stability and Photoluminescence Retention of Lead-Based Quantum Dots in Saline Buffers and Biological Media through Surface Modification. Chem. Mater. 2013, 25, 2035–2044. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Frank, C.W. Grafting of Poly(γ-benzyl-l-glutamate) on Chemically Modified Silicon Oxide Surfaces. Langmuir 1996, 12, 5824–5829. [Google Scholar] [CrossRef]
- Shen, X.C.; Fang, X.Z.; Zhou, Y.H.; Liang, H. Synthesis and Characterization of 3-Aminopropyltriethoxysilane-Modified Superparamagnetic Magnetite Nanoparicles. Chem. Lett. 2004, 33, 1468–1469. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, L.; Liu, W.; Song, Z. Surface modification of ceria nanoparticles and their chemical mechanical polishing behavior on glass substrate. Appl. Surf. Sci. 2010, 256, 3856–3861. [Google Scholar] [CrossRef]
- Zheng, S.; Guan, Y.; Yu, H.; Huang, G.; Zheng, C. Poly-l-lysine-coated PLGA/poly(amino acid)-modified hydroxyapatite porous scaffolds as efficient tissue engineering scaffolds for cell adhesion, proliferation, and differentiation. New J. Chem. 2019, 43, 9989–10002. [Google Scholar] [CrossRef]
- Li, L.; Shi, X.; Wang, Z.; Wang, Y.; Jiao, Z.; Zhang, P. In situ polymerization of poly(γ-benzyl-l-glutamate) on mesoporous hydroxyapatite with high graft amounts for the direct fabrication of biodegradable cell microcarriers and their osteogenic induction. J. Mater. Chem. B 2018, 6, 3315–3330. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Qin, L.; Li, H.; Liang, W. Magnetic coagulation and flocculation of a kaolin suspension using Fe3O4 coated with SiO2. J. Environ. Chem. Eng. 2021, 9, 105980. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; You, X.; Xiao, Q. Synthesis of mesoporous core-shell structured GdPO4:Eu@SiO2@mSiO2 nanorods for drug delivery and cell imaging applications. J. Rare Earths 2020, 38, 1086–1092. [Google Scholar] [CrossRef]
- Wei, J.; Liu, A.; Chen, L.; Zhang, P.; Chen, X.; Jing, X. The Surface Modification of Hydroxyapatite Nanoparticles by the Ring Opening Polymerization of γ-Benzyl-L-glutamate N-carboxyanhydride. Macromol. Biosci. 2009, 9, 631–638. [Google Scholar] [CrossRef]
- Yu, M.; Wang, H.; Lin, C.K.; Li, G.Z.; Lin, J. Sol–gel synthesis and photoluminescence properties of spherical SiO2@LaPO4:Ce3+/Tb3+ particles with a core–shell structure. Nanotechnology 2006, 17, 3245. [Google Scholar] [CrossRef]
- Yu, X.; Liu, X.; Wu, W.; Yang, K.; Mao, R.; Ahmad, F.; Chen, X.; Li, W. CT/MRI-Guided Synergistic Radiotherapy and X-ray Inducible Photodynamic Therapy Using Tb-Doped Gd-W-Nanoscintillators. Angew. Chem. Int. Ed. 2019, 58, 2017–2022. [Google Scholar] [CrossRef]
- Choi, J.-s.; Lee, J.-H.; Shin, T.-H.; Song, H.-T.; Kim, E.Y.; Cheon, J. Self-Confirming “AND” Logic Nanoparticles for Fault-Free MRI. J. Am. Chem. Soc. 2010, 132, 11015–11017. [Google Scholar] [CrossRef] [Green Version]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. The rare earth element (REE) lanthanum (La) induces hormesis in plants. Environ. Pollut. 2018, 238, 1044–1047. [Google Scholar] [CrossRef]
- Baipaywad, P.; Hong, S.V.; Kim, J.B.; Hwang, J.; Choi, J.; Park, H.; Paik, T. Single-step acid-catalyzed synthesis of luminescent colloidal organosilica nanobeads. Nano Converg. 2022, 9, 12. [Google Scholar] [CrossRef]
- Sun, M.; Wang, H.; Li, X. Modification of cellulose microfibers by polyglutamic acid and mesoporous silica nanoparticles for Enterovirus 71 adsorption. Mater. Lett. 2020, 277, 128320. [Google Scholar] [CrossRef]
- Dhavale, R.P.; Waifalkar, P.P.; Sharma, A.; Dhavale, R.P.; Sahoo, S.C.; Kollu, P.; Chougale, A.D.; Zahn, D.R.T.; Salvan, G.; Patil, P.S.; et al. Monolayer grafting of aminosilane on magnetic nanoparticles: An efficient approach for targeted drug delivery system. J. Colloid Interface Sci. 2018, 529, 415–425. [Google Scholar] [CrossRef]
- Dadej, A.; Woźniak-Braszak, A.; Bilski, P.; Piotrowska-Kempisty, H.; Józkowiak, M.; Geszke-Moritz, M.; Moritz, M.; Dadej, D.; Jelińska, A. Modification of the Release of Poorly Soluble Sulindac with the APTES-Modified SBA-15 Mesoporous Silica. Pharmaceutics 2021, 13, 693. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Crespo, J.J.; Deladriere, C.; Nebot, V.J.; Charbonnier, D.; Masiá, E.; Paul, A.; James, C.; Armiñán, A.; Vicent, M.J. Anticancer Activity Driven by Drug Linker Modification in a Polyglutamic Acid-Based Combination-Drug Conjugate. Adv. Funct. Mater. 2018, 28, 1800931. [Google Scholar] [CrossRef] [Green Version]
- Quan, B.D.; Wojtas, M.; Sone, E.D. Polyaminoacids in Biomimetic Collagen Mineralization: Roles of Isomerization and Disorder in Polyaspartic and Polyglutamic Acids. Biomacromolecules 2021, 22, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Ramakrishna, S. Precipitation of nanohydroxyapatite on PLLA/PBLG/Collagen nanofibrous structures for the differentiation of adipose derived stem cells to osteogenic lineage. Biomaterials 2012, 33, 846–855. [Google Scholar] [CrossRef]
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef]
- Datta, P.; Ghosh, P.; Ghosh, K.; Maity, P.; Samanta, S.K.; Ghosh, S.K.; Mohapatra, P.K.D.; Chatterjee, J.; Dhara, S.J. In Vitro ALP and Osteocalcin Gene Expression Analysis and In Vivo Biocompatibility of N-Methylene Phosphonic Chitosan Nanofibers for Bone Regeneration. J. Biomed. Nanotechnol. 2013, 9, 870–879. [Google Scholar] [CrossRef]
- Chiu, L.-H.; Lai, W.-F.T.; Chang, S.-F.; Wong, C.-C.; Fan, C.-Y.; Fang, C.-L.; Tsai, Y.-H. The effect of type II collagen on MSC osteogenic differentiation and bone defect repair. Biomaterials 2014, 35, 2680–2691. [Google Scholar] [CrossRef]
- Zhou, P.; Xia, D.; Ni, Z.; Ou, T.; Wang, Y.; Zhang, H.; Mao, L.; Lin, K.; Xu, S.; Liu, J. Calcium silicate bioactive ceramics induce osteogenesis through oncostatin M. Bioact. Mater. 2021, 6, 810–822. [Google Scholar] [CrossRef]
- Bunpetch, V.; Zhang, X.; Li, T.; Lin, J.; Maswikiti, E.P.; Wu, Y.; Cai, D.; Li, J.; Zhang, S.; Wu, C.; et al. Silicate-based bioceramic scaffolds for dual-lineage regeneration of osteochondral defect. Biomaterials 2019, 192, 323–333. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, C.; Yang, X.; Peng, F.; Huang, Y.; He, Y. A SiO2 layer on PEO-treated Mg for enhanced corrosion resistance and bone regeneration. Front. Bioeng. Biotechnol. 2022, 23, 944. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon: A Possible Factor in Bone Calcification. Science 1970, 167, 279–280. [Google Scholar] [CrossRef]
- Götz, W.; Tobiasch, E.; Witzleben, S.; Schulze, M. Effects of Silicon Compounds on Biomineralization, Osteogenesis, and Hard Tissue Formation. Pharmaceutics 2019, 11, 117. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Feng, L.; Chen, B.; Fu, B.; Zhu, M. The role of rare earth elements in bone tissue engineering scaffolds—A review. Compos. Part B Eng. 2022, 235, 109758. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Y.; Qian, Z. Nanostructured Surface Modification to Bone Implants for Bone Regeneration. J. Biomed. Nanotechnol. 2018, 14, 628–648. [Google Scholar] [CrossRef]

















| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| mGdPO4·H2O:VTEOS (mg:vL) | 4:5 | 1:1 | 5:4 | 5:3 | 5:2 |
| Zeta Potential (mV) | −26.52 | −30.69 | −16.98 | −16.96 | −12.71 |
| GdPO4·H2O@SiO2:APS | 1:0.22 | |||
| GdPO4·H2O/APS:NCA | 1:0.125 | 1:0.25 | 1:0.5 | 1:1 |
| Graft rate of GdPO4·H2O/PBLG (%) | 4.14% | 5.93% | 13.72% | 15.93% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Guo, Z.; Yang, C.; Wang, F.; Zhang, P.; Wang, Y.; Guo, M.; Wang, Z.; Huang, J.; Zhang, L. Surface Biofunctionalization of Gadolinium Phosphate Nanobunches for Boosting Osteogenesis/Chondrogenesis Differentiation. Int. J. Mol. Sci. 2023, 24, 2032. https://doi.org/10.3390/ijms24032032
Cai Z, Guo Z, Yang C, Wang F, Zhang P, Wang Y, Guo M, Wang Z, Huang J, Zhang L. Surface Biofunctionalization of Gadolinium Phosphate Nanobunches for Boosting Osteogenesis/Chondrogenesis Differentiation. International Journal of Molecular Sciences. 2023; 24(3):2032. https://doi.org/10.3390/ijms24032032
Chicago/Turabian StyleCai, Zhongxing, Ziyi Guo, Chaohui Yang, Fei Wang, Peibiao Zhang, Yu Wang, Min Guo, Zongliang Wang, Jing Huang, and Long Zhang. 2023. "Surface Biofunctionalization of Gadolinium Phosphate Nanobunches for Boosting Osteogenesis/Chondrogenesis Differentiation" International Journal of Molecular Sciences 24, no. 3: 2032. https://doi.org/10.3390/ijms24032032
APA StyleCai, Z., Guo, Z., Yang, C., Wang, F., Zhang, P., Wang, Y., Guo, M., Wang, Z., Huang, J., & Zhang, L. (2023). Surface Biofunctionalization of Gadolinium Phosphate Nanobunches for Boosting Osteogenesis/Chondrogenesis Differentiation. International Journal of Molecular Sciences, 24(3), 2032. https://doi.org/10.3390/ijms24032032