Actinomycin-X2-Immobilized Silk Fibroin Film with Enhanced Antimicrobial and Wound Healing Activities
Abstract
:1. Introduction
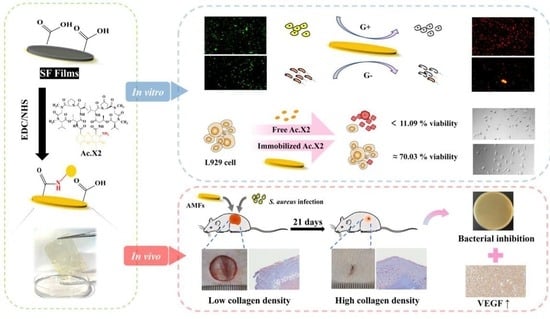
2. Results and Discussion
2.1. Preparation of Actinomycin-X2-Immobilized Silk Fibroin Film
2.2. Characterization of AMFs
2.3. Evaluation of Antibacterial Activity
2.4. Bactericidal Mechanisms of AMFs
2.5. Evaluation of Biocompatibility
2.6. Evaluation of the Stability of AMFs
2.7. In Vivo Evaluation of Wound Healing
2.7.1. Evaluation of Full-Thickness Skin Infected Wound Model
2.7.2. Histological and Immunohistochemical (IHC) Evaluation
3. Materials and Methods
3.1. Materials
3.2. Preparation of AMFs
3.2.1. Preparation of SF Films
3.2.2. Preparation of Purified Ac.X2
3.2.3. Preparation of the Ac.X2-Immobilized SF Film
3.3. Characterizations of AMFs
3.3.1. Determination of Immobilized Peptide Density
3.3.2. Analytical Methods of Characterization
3.4. Antimicrobial Activity Assessment
3.4.1. Bacteriostatic Method
3.4.2. Long-Term Antimicrobial Stability Test
3.5. Biocompatibility Assessment
3.5.1. Brine Shrimp Culture
3.5.2. Cell Culture
3.6. Assessment of Leaches
3.7. In Vivo Wound Healing Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Atefyekta, S.; Pihl, M.; Lindsay, C.; Heilshorn, S.C.; Andersson, M. Antibiofilm elastin-like polypeptide coatings: Functionality, stability, and selectivity. Acta Biomater. 2019, 83, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Du Vigneaud, V.; Ressler, C.; Swan, C.J.M.; Roberts, C.W.; Katsoyannis, P.G.; Gordon, S. The synthesis of an octapeptide amide with the hormonal activity of oxytocin. J. Am. Chem. Soc. 1953, 75, 4879–4880. [Google Scholar] [CrossRef]
- Du Vigneaud, V.; Gish, D.T.; Katsoyannis, P.G.; Hess, G.P. Synthesis of the Pressor-Antidiuretic Hormone, Arginine-Vasopressin. J. Am. Chem. Soc. 1958, 80, 3355–3358. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, K.K.; Sharma, A.; Jain, R. Peptide-based drug discovery: Current status and recent advances. Drug Discov. Today 2023, 28, 103464. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef]
- Gunasekera, S.; Muhammad, T.; Strömstedt, A.A.; Rosengren, K.J.; Göransson, U. Backbone Cyclization and Dimerization of LL-37-Derived Peptides Enhance Antimicrobial Activity and Proteolytic Stability. Front. Microbiol. 2020, 11, 168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Woodruff, S.A.; Woodruff, H.B. Bacteriostatic and bactericidal substances produd by a soil actinomyces. Proc. Soc. Exp. Biol. Med. 1940, 45, 609. [Google Scholar]
- Morgan, T.H. Cross- and Self-fertilization in the ascidian molgula manhattensis. Biol. Bull. 1942, 82, 172–177. [Google Scholar] [CrossRef]
- Chen, F.; Sha, F.; Chin, K.; Chou, S.-H. Unique actinomycin D binding to self-complementary d(CXYGGCCY′X′G) sequences: Duplex disruption and binding to a nominally base-paired hairpin. Nucleic Acids Res. 2003, 31, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Ye, K.; Zhu, X.; Zhang, H.; Si, R.; Chen, J.; Chen, Z.; Song, K.; Yu, Z.; Han, B. Actinomycin X2, an Antimicrobial Depsipeptide from Marine-Derived Streptomyces cyaneofuscatus Applied as a Good Natural Dye for Silk Fabric. Mar. Drugs 2021, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Khanjanzadeh, H.; Park, B.-D.; Pirayesh, H. Intelligent pH- and ammonia-sensitive indicator films using neutral red immobilized onto cellulose nanofibrils. Carbohydr. Polym. 2022, 296, 119910. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; He, F.; Li, Y.; Fang, L.; Wang, K.; Song, J.; Zhou, J.; Li, Q.; Zhang, H. Cytotoxic antibiotic angucyclines and actinomycins from the Streptomyces sp. XZHG99T. J. Antibiot. 2018, 71, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, K.A.; Bholay, A.D.; Rai, P.K.; Mohammed, H.A.; Khan, R.A.; Azam, F.; Jaremko, M.; Emwas, A.H.; Stefanowicz, P.; Waliczek, M.; et al. Isolation, characterization, anti-MRSA evaluation, and in-silico multi-target anti-microbial validations of actinomycin X(2) and actinomycin D produced by novel Streptomyces smyrnaeus UKAQ_23. Sci. Rep. 2021, 11, 14539. [Google Scholar] [CrossRef]
- Sharma, M.; Manhas, R.K. Purification and characterization of actinomycins from Streptomyces strain M7 active against methicillin resistant Staphylococcus aureus and vancomycin resistant Enterococcus. BMC Microbiol. 2019, 19, 44. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Song, F.; Wang, Q.; Abdel-Mageed, W.M.; Guo, H.; Fu, C.; Hou, W.; Dai, H.; Liu, X.; Yang, N.; et al. A marine-derived Streptomyces sp. MS449 produces high yield of actinomycin X2 and actinomycin D with potent anti-tuberculosis activity. Appl. Microbiol. Biotechnol. 2012, 95, 919–927. [Google Scholar] [CrossRef]
- Kurosawa, K.; Bui, V.P.; Vanessendelft, J.L.; Willis, L.B.; Lessard, P.A.; Ghiviriga, I.; Sambandan, T.G.; Rha, C.K.; Sinskey, A.J. Characterization of Streptomyces MITKK-103, a newly isolated actinomycin X2-producer. Appl. Microbiol. Biotechnol. 2006, 72, 145–154. [Google Scholar] [CrossRef]
- Lim, K.; Chua, R.R.Y.; Ho, B.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S.J. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef]
- Saravanan, R.; Li, X.; Lim, K.; Mohanram, H.; Peng, L.; Mishra, B.; Basu, A.; Lee, J.-M.; Bhattacharjya, S.; Leong, S.S.J. Design of short membrane selective antimicrobial peptides containing tryptophan and arginine residues for improved activity, salt-resistance, and biocompatibility. Biotechnol. Bioeng. 2014, 111, 37–49. [Google Scholar] [CrossRef]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef] [Green Version]
- Andrade, C.A.; Silva, R.R.; Avelino, K.Y.; Ribeiro, K.L.; Franco, O.L.; Oliveira, M.D. Chemical immobilization of antimicrobial peptides on biomaterial surfaces. Front. Biosci. 2016, 8, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Schwarze, H.; Kuntscher, M.; Uhlig, C.; Hierlemann, H.; Prantl, L.; Ottomann, C.; Hartmann, B. Suprathel, a new skin substitute, in the management of partial-thickness burn wounds: Results of a clinical study. Ann. Plast. Surg. 2008, 60, 181–185. [Google Scholar] [CrossRef]
- Bai, L.; Zhu, L.; Min, S.; Liu, L.; Cai, Y.; Yao, J. Surface modification and properties of Bombyx mori silk fibroin films by antimicrobial peptide. Appl. Surf. Sci. 2008, 254, 2988–2995. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, F.; Torculas, M.; Lofland, S.E.; Hu, X. Formic Acid Regenerated Mori, Tussah, Eri, Thai, and Muga Silk Materials: Mechanism of Self-Assembly. ACS Biomater. Sci. Eng. 2019, 5, 6361–6373. [Google Scholar] [CrossRef]
- Nakanishi, K. Infrared absorption spectroscopy—Practical. J. Mol. Struct. 1970, 5, 244. [Google Scholar]
- Zhang, J.; Rajkhowa, R.; Li, J.; Liu, X.; Wang, X. Silkworm cocoon as natural material and structure for thermal insulation. Mater. Des. 2013, 49, 842–849. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Z. Microstructure Transitions and Dry-Wet Spinnability of Silk Fibroin Protein from Waste Silk Quilt. Polymers 2019, 11, 1622. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Li, J.; Zhang, S.; Yin, Z.; Xing, T.; Kaplan, D.L. The influence of the hydrophilic–lipophilic environment on the structure of silk fibroin protein. J. Mater. Chem. B 2015, 3, 2599–2606. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Cai, J.-Y. Enhanced cell affinity of poly(l-lactic acid) modified by base hydrolysis: Wettability and surface roughness at nanometer scale. Curr. Appl. Phys. 2007, 7, e108–e111. [Google Scholar] [CrossRef]
- Groth, T. Surfaces modification of biomaterials to control adhesion of cells. Biomed. Pharmacother. 2006, 60, 468–469. [Google Scholar] [CrossRef]
- Khang, G.; Choee, J.-H.; Rhee, J.M.; Lee, H.B. Interaction of different types of cells on physicochemically treated poly(L-lactide-co-glycolide) surfaces. J. Appl. Polym. Sci. 2002, 85, 1253–1262. [Google Scholar] [CrossRef]
- Siboni, R.B.; Nakamori, M.; Wagner, S.D.; Struck, A.J.; Coonrod, L.A.; Harriott, S.A.; Cass, D.M.; Tanner, M.K.; Berglund, J.A. Actinomycin D Specifically Reduces Expanded CUG Repeat RNA in Myotonic Dystrophy Models. Cell Rep. 2015, 13, 2386–2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 2002, 28, 988–994. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, S.; Feng, H.; Wang, Y.; Li, S.; Zhou, X.; Wang, M.; Rei, L. Antifouling performance of in situ synthesized chitosan-zinc oxide hydrogel film against alga M. aeruginosa. Int. J. Biol. Macromol. 2022, 200, 234–241. [Google Scholar] [CrossRef]
- Zhang, K.; Zheng, H.; Liang, S.; Gao, C. Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth. Acta Biomater. 2016, 37, 131–142. [Google Scholar] [CrossRef]
- Song, D.W.; Kim, S.H.; Kim, H.H.; Lee, K.H.; Ki, C.S.; Park, Y.H. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: Implications for wound healing. Acta Biomater. 2016, 39, 146–155. [Google Scholar] [CrossRef]
- Sun, F.; Hu, W.; Zhao, Y.; Li, Y.; Xu, X.; Li, Y.; Zhang, H.; Luo, J.; Guo, B.; Ding, C.; et al. Invisible assassin coated on dental appliances for on-demand capturing and killing of cariogenic bacteria. Colloids Surf. B Biointerfaces 2022, 217, 112696. [Google Scholar] [CrossRef]
- Kadakia, P.U.; Jain, E.; Hixon, K.R.; Eberlin, C.T.; Sell, S.A. Sonication induced silk fibroin cryogels for tissue engineering applications. Mater. Res. Express 2016, 3, 055401. [Google Scholar] [CrossRef]
- Liu, W.; Carlisle, C.R.; Sparks, E.A.; Guthold, M. The mechanical properties of single fibrin fibers. J. Thromb. Haemost. 2010, 8, 1030–1036. [Google Scholar] [CrossRef] [Green Version]
- Panico, A.; Paladini, F.; Pollini, M. Development of regenerative and flexible fibroin-based wound dressings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Chen, C.; Zhu, S.; Zhu, M.; Dai, J.; Ray, U.; Li, Y.; Kuang, Y.; Li, Y.; Quispe, N.; et al. Processing bulk natural wood into a high-performance structural material. Nature 2018, 554, 224–228. [Google Scholar] [CrossRef]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C.L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Kreve, S.; dos Reis, A.C. Influence of the electrostatic condition of the titanium surface on bacterial adhesion: A systematic review. J. Prosthet. Dent. 2021, 125, 416–420. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, D.; He, Y.; Zhao, X.; Bai, R. Modification of membrane surface for anti-biofouling performance: Effect of anti-adhesion and anti-bacteria approaches. J. Membr. Sci. 2010, 346, 121–130. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Bisno, A.L. Molecular aspects of bacterial colonization. Infect. Control Hosp. Epidemiol. 1995, 16, 648–657. [Google Scholar] [CrossRef]
- Lakes, A.L.; Peyyala, R.; Ebersole, J.L.; Puleo, D.A.; Hilt, J.Z.; Dziubla, T.D. Synthesis and Characterization of an Antibacterial Hydrogel Containing Covalently Bound Vancomycin. Biomacromolecules 2014, 15, 3009–3018. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Speziale, P.; Arciola, C.R. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials 2010, 31, 6363–6377. [Google Scholar] [CrossRef]
- Haynie, S.L.; Crum, G.A.; Doele, B.A. Antimicrobial activities of amphiphilic peptides covalently bonded to a water-insoluble resin. Antimicrob. Agents Chemother. 1995, 39, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagheri, M.; Beyermann, M.; Dathe, M. Immobilization Reduces the Activity of Surface-Bound Cationic Antimicrobial Peptides with No Influence upon the Activity Spectrum. Antimicrob. Agents Chemother. 2009, 53, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.E.; Im, S.; Kim, H.; Sohn, J.-H.; Cho, B.-K.; Cho, J.H.; Sung, B.H.; Kim, S.C. Adhesive Antimicrobial Peptides Containing 3,4-Dihydroxy-L-Phenylalanine Residues for Direct One-Step Surface Coating. Int. J. Mol. Sci. 2021, 22, 11915. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, L.; Zhang, X.; Yang, J.; Chen, X.; Li, F.; Liu, J.; Huang, R. Multi-Omics Analysis Reveals Anti-Staphylococcus aureus Activity of Actinomycin D Originating from Streptomyces parvulus. Int. J. Mol. Sci. 2021, 22, 12231. [Google Scholar] [CrossRef]
- Dwivedi, S.; Wahab, R.; Khan, F.; Mishra, Y.K.; Musarrat, J.; Al-Khedhairy, A.A. Reactive Oxygen Species Mediated Bacterial Biofilm Inhibition via Zinc Oxide Nanoparticles and Their Statistical Determination. PLoS ONE 2014, 9, e111289. [Google Scholar] [CrossRef]
- Messner, K.R.; Imlay, J.A. The Identification of Primary Sites of Superoxide and Hydrogen Peroxide Formation in the Aerobic Respiratory Chain and Sulfite Reductase Complex of Escherichia coli. J. Biol. Chem. 1999, 274, 10119–10128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosio, P.J.S.; Tronnet, A.; Verhaeghe, P.; Bonduelle, C. Synthetic Polypeptide Polymers as Simplified Analogues of Antimicrobial Peptides. Biomacromolecules 2021, 22, 57–75. [Google Scholar] [CrossRef]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals Radii for the Whole Main Group. J. Phys. Chem. A 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.-Z.; Xie, Z.-X.; Zhou, Z.-H. 70 Years of Crystallographic van der Waals Radii. Acta Phys. Chim. Sin. 2010, 26, 1795–1800. [Google Scholar]
- Li, J.; Koh, J.-J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane Active Antimicrobial Peptides: Translating Mechanistic Insights to Design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Si, R.; Chen, W.; Chen, J.; Yang, Y.; Zhou, W.; Zhang, Q.; Chen, C.; Han, B. Green chemistry fabrication of durable antimicrobial peptide-immobilized silk fibroin films for accelerated full-thickness wound healing. Mater. Today Chem. 2023, 29, 101468. [Google Scholar] [CrossRef]
- Baran, E.T.; Tuzlakoğlu, K.; Mano, J.F.; Reis, R.L. Enzymatic degradation behavior and cytocompatibility of silk fibroin–starch–chitosan conjugate membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 1314–1322. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Liu, H.; Li, Y.; Li, Y.; Ma, Q.; Zhang, J.; Hu, X. Tunable Biodegradable Polylactide–Silk Fibroin Scaffolds Fabricated by a Solvent-Free Pressure-Controllable Foaming Technology. ACS Appl. Bio Mater. 2020, 3, 8795–8807. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Morais, A.D.S.; Maia, F.R.; Canadas, R.F.; Costa, J.B.; Oliveira, A.L.; Oliveira, J.M.; Reis, R.L. Combinatory approach for developing silk fibroin scaffolds for cartilage regeneration. Acta Biomater. 2018, 72, 167–181. [Google Scholar] [CrossRef]
- Sun, F.; Xiao, D.; Su, H.; Chen, Z.; Wang, B.; Feng, X.; Mao, Z.; Sui, X. Highly stretchable porous regenerated silk fibroin film for enhanced wound healing. J. Mater. Chem. B 2023, 11, 1486–1494. [Google Scholar] [CrossRef]
- Yang, Q.; Xie, Z.; Hu, J.; Liu, Y. Hyaluronic acid nanofiber mats loaded with antimicrobial peptide towards wound dressing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112319. [Google Scholar] [CrossRef]
- Benedetto, A.; Cassone, A.; Delfini, C. Resistance of African Green Monkey Kidney Cell Lines to Actinomycin D: Drug Uptake in 37 RC Cells After Persistent Inhibition of Transcription. Antimicrob. Agents Chemother. 1979, 15, 300–312. [Google Scholar] [CrossRef] [Green Version]
- Madhavarao, M.S.; Chaykovsky, M.; Sengupta, S.K. N7-Substituted 7-aminoactinomycin D analogues. Synthesis and biological properties. J. Med. Chem. 1978, 21, 958–961. [Google Scholar] [CrossRef]
- Mauro, N.; Schillaci, D.; Varvarà, P.; Cusimano, M.G.; Geraci, D.M.; Giuffrè, M.; Cavallaro, G.; Maida, C.M.; Giammona, G. Branched High Molecular Weight Glycopolypeptide with Broad-Spectrum Antimicrobial Activity for the Treatment of Biofilm Related Infections. ACS Appl. Mater. Interfaces 2017, 10, 318–331. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhou, G.; Chen, Y.; Mao, C.; Yang, M. Polydopamine-Coated Antheraea pernyi (A. pernyi) Silk fibroin films promote cell adhesion and wound healing in skin tissue repair. ACS Appl. Mater. Interf. 2019, 11, 34736–34743. [Google Scholar] [CrossRef]
- Sun, S.; Hao, M.; Ding, C.; Zhang, J.; Ding, Q.; Zhang, Y.; Zhao, Y.; Liu, W. SF/PVP nanofiber wound dressings loaded with phlorizin: Preparation, characterization, in vivo and in vitro evaluation. Colloids Surf. B Biointerfaces 2022, 217, 112692. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Xu, X.; Zhang, L.; Liu, Y.; Liu, S.; Liu, Z.; Wu, M.; Shuai, Q. Self-healing/pH-responsive/inherently antibacterial polysaccharide-based hydrogel for a photothermal strengthened wound dressing. Colloids Surf. B Biointerfaces 2022, 218, 112738. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.R.; Huang, K.; Zhao, J.; Zhu, T.; Morsi, Y.; Aldalbahi, A.; El-Newehy, M.; Yan, X.; Mo, X. PLCL/Silk fibroin based antibacterial nano wound dressing encapsulating oregano essential oil: Fabrication, characterization and biological evaluation. Colloid Surf. B Biointerfaces 2020, 196, 111352. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.R.; Sultan, M.T.; Park, H.J.; Lee, J.M.; Ju, H.W.; Lee, O.J.; Lee, D.J.; Kaplan, D.L.; Park, C.H. NF-kappaB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 2018, 67, 183–195. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, H.; Zhou, L.; Cheng, F.; Liu, Z.; Zhang, H.; Wang, L.; Zhang, Q. Nanoenzyme-Reinforced Injectable Hydrogel for Healing Diabetic Wounds Infected with Multidrug Resistant Bacteria. Nano Lett. 2020, 20, 5149–5158. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Huang, J.; Wu, J. Novel Glucose-Responsive Antioxidant Hybrid Hydrogel for Enhanced Diabetic Wound Repair. ACS Appl. Mater. Interfaces 2022, 14, 7680–7689. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Z.; Zhao, H.; Cui, H.; Shen, J.; Chang, J.; Li, H.; He, Y. Bioactive Injectable Hydrogels Containing Desferrioxamine and Bioglass for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 30103–30114. [Google Scholar] [CrossRef]
- Keswani, S.G.; Balaji, S.; Le, L.D.; Leung, A.; Parvadia, J.K.; Frischer, J.; Yamano, S.; Taichman, N.; Crombleholme, T.M. Role of salivary vascular endothelial growth factor (VEGF) in palatal mucosal wound healing. Wound Repair Regen. 2013, 21, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.; Cui, Y.; Liang, Y.; Liu, H.; Ma, B.; Ge, S. Unilateral Silver-Loaded Silk Fibroin Difunctional Membranes as Antibacterial Wound Dressings. ACS Omega 2021, 6, 17555–17565. [Google Scholar] [CrossRef]









| Sample | Immobilization Density (μg/cm2) | Immobilized Ac.X2 # (μg) | Free Ac.X2 Concentration # (μg/mL) | Amount of Leachates (ng/cm2) | Concentration of Leachates ## (ng/mL) |
|---|---|---|---|---|---|
| A-5 | 0.52 | 0.29 | 2.9 | 4.492 | 25.39 |
| A-10 | 0.96 | 0.54 | 5.4 | 8.852 | 50.03 |
| A-20 | 1.67 | 0.95 | 9.5 | 10.856 | 61.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Xie, Z.; Si, R.; Chen, Z.; Javeed, A.; Li, J.; Wu, Y.; Han, B. Actinomycin-X2-Immobilized Silk Fibroin Film with Enhanced Antimicrobial and Wound Healing Activities. Int. J. Mol. Sci. 2023, 24, 6269. https://doi.org/10.3390/ijms24076269
Zhou W, Xie Z, Si R, Chen Z, Javeed A, Li J, Wu Y, Han B. Actinomycin-X2-Immobilized Silk Fibroin Film with Enhanced Antimicrobial and Wound Healing Activities. International Journal of Molecular Sciences. 2023; 24(7):6269. https://doi.org/10.3390/ijms24076269
Chicago/Turabian StyleZhou, Wenjing, Zhenxia Xie, Ranran Si, Zijun Chen, Ansar Javeed, Jiaxing Li, Yang Wu, and Bingnan Han. 2023. "Actinomycin-X2-Immobilized Silk Fibroin Film with Enhanced Antimicrobial and Wound Healing Activities" International Journal of Molecular Sciences 24, no. 7: 6269. https://doi.org/10.3390/ijms24076269
APA StyleZhou, W., Xie, Z., Si, R., Chen, Z., Javeed, A., Li, J., Wu, Y., & Han, B. (2023). Actinomycin-X2-Immobilized Silk Fibroin Film with Enhanced Antimicrobial and Wound Healing Activities. International Journal of Molecular Sciences, 24(7), 6269. https://doi.org/10.3390/ijms24076269