Valorisation of Whey Permeate in Sequential Bioprocesses towards Value-Added Products–Optimisation of Biphasic and Classical Batch Cultures of Kluyveromyces marxianus
Abstract
:1. Introduction
2. Results
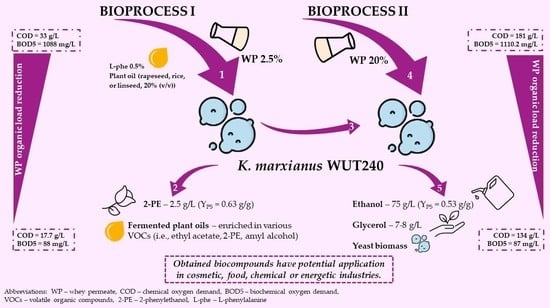
2.1. Whey Permeate Valorisation Enables 2-Phenylethanol and Fermented Plant Oils Production–Study on Bioprocess I
2.2. Efficient Management of Whey Permeate with K. marxianus WUT240 Strain Is Possible in a Laboratory-Scale Bioreactor
2.3. Use of K. marxianus WUT240 Biomass Enables Efficient Fermentation of Lactose to Ethanol in Cheese Whey Permeate–Study on Bioprocess II
- yeast biomass is metabolically active and can be directly used for alcoholic fermentation;
- the gentle shaking does not significantly affect the final ethanol titer;
- yeast biomass originating from two-phase cultures generally allows obtaining higher ethanol titer and YP/X values than from control cultures.
- direct use of freeze-dried yeast;
- utilisation of yeast preincubated in a SAB medium for 4 h at 30 °C.
2.4. Whey Permeate Bioconversion with K. marxianus WUT240 Leads to Its Organic Load Reduction
3. Discussion
4. Materials and Methods
4.1. Yeast Strain and Media Composition
4.2. Effect of Exogenous Lactose, Ethanol and Ethyl Acetate on K. marxianus WUT240 Growth
4.3. Experimental Design
4.3.1. 2-Phenylethanol Production in Two-Phase Batch Cultures in Shaking Flasks (Bioprocess I)
4.3.2. Bioprocess I in 4.8-L Laboratory Bioreactor Scale
4.3.3. Fermentation of Lactose to Ethanol (Bioprocess II)
- (I)
- For the determination of yeast biomass activity after Bioprocess I, the entire volume of two-phase cultures was centrifuged (5000× g for 5 min, 20 °C), the supernatant was separated, and the biomass was washed twice with sterile water. The biomass thus prepared was suspended in 150 mL of WP200 medium in 300 mL Erlenmeyer flasks and incubated at 30 °C, without or with gentle shaking (50 rpm), for 72 h.
- (II)
- In order to determine the effect of initial biomass concentration on fermentation, the biomass multiplied in Bioprocess I was freeze-dried and subsequently weighed to obtain the selected inoculum concentration (0.5–4%, w/v). Two variants were tested, without and with prior revival (SAB medium, 30 °C, 240 rpm for 4 h) of lyophilised yeast. 75 mL cultures in 250 mL Erlenmeyer flasks were incubated stationary at 30 °C for 72 h.
4.4. COD and BOD5 Measurements
4.5. Analytical Methods
4.6. Calculations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navarrete, C.; Martínez, J.L. Non-Conventional Yeasts as Superior Production Platforms for Sustainable Fermentation Based Bio-Manufacturing Processes. AIMS Bioeng. 2020, 7, 289–305. [Google Scholar] [CrossRef]
- Banat, I.M.; Nigam, P.; Marchant, R. Isolation of Thermotolerant, Fermentative Yeasts Growing at 52 °C and Producing Ethanol at 45 °C and 50 °C. World J. Microbiol. Biotechnol. 1992, 8, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, G.G.; Heinzle, E.; Wittmann, C.; Gombert, A.K. The Yeast Kluyveromyces marxianus and Its Biotechnological Potential. Appl. Microbiol. Biotechnol. 2008, 79, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Morrissey, J.P. Kluyveromyces marxianus: A Yeast Emerging from Its Sister’s Shadow. Fungal Biol. Rev. 2010, 24, 17–26. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Wang, D.; Gao, X.; Hong, J. Xylitol Production at High Temperature by Engineered Kluyveromyces marxianus. Bioresour. Technol. 2014, 152, 192–201. [Google Scholar] [CrossRef]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces marxianus: An Emerging Yeast Cell Factory for Applications in Food and Biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef]
- Aksu, Z.; Dönmez, G. The Use of Molasses in Copper(II) Containing Wastewaters: Effects on Growth and Copper(II) Bioaccumulation Properties of Kluyveromyces marxianus. Process Biochem. 2000, 36, 451–458. [Google Scholar] [CrossRef]
- Whey Permeate Market Information by Application, and Region—Forecast till 2028. Research Report. Market Research Future. 2022. Available online: https://www.globenewswire.com/en/news-release/2022/05/04/2435465/0/en/Whey-Permeate-Market-Size-Worth-USD-970-6-Million-by-2028-Witnessing-a-CAGR-of-4-05-Report-by-Market-Research-Future-MRFR.html (accessed on 15 January 2023).
- Bentahar, J.; Doyen, A.; Beaulieu, L.; Deschênes, J.S. Acid Whey Permeate: An Alternative Growth Medium for Microalgae Tetradesmus Obliquus and Production of β-Galactosidase. Algal Res. 2019, 41, 101559. [Google Scholar] [CrossRef]
- Pasotti, L.; Zucca, S.; Casanova, M.; Micoli, G.; Cusella De Angelis, M.G.; Magni, P. Fermentation of Lactose to Ethanol in Cheese Whey Permeate and Concentrated Permeate by Engineered Escherichia coli. BMC Biotechnol. 2017, 17, 48. [Google Scholar] [CrossRef]
- Krischke, W.; Schröder, M.; Trösch, W. Continuous Production of L-Lactic Acid from Whey Permeate by Immobilized Lactobacillus casei Subsp. casei. Appl. Microbiol. Biotechnol. 1991, 34, 573–578. [Google Scholar] [CrossRef]
- Etschmann, M.; Bluemke, W.; Sell, D.; Schrader, J. Biotechnological Production of 2-Phenylethanol. Appl. Microbiol. Biotechnol. 2002, 59, 1–8. [Google Scholar] [CrossRef]
- Martínez, O.; Sánchez, A.; Font, X. Bioproduction of 2-Phenylethanol and 2-Phenethyl Acetate by Kluyveromyces marxianus through the Solid-State Fermentation of Sugarcane Bagasse. Appl. Microbiol. Biotechnol. 2018, 102, 4703–4716. [Google Scholar] [CrossRef]
- Güneşer, O.; Karagül-Yüceer, Y.; Wilkowska, A.; Kregiel, D. Volatile Metabolites Produced from Agro-Industrial Wastes by Na-Alginate Entrapped Kluyveromyces marxianus. Braz. J. Microbiol. 2016, 47, 965–972. [Google Scholar] [CrossRef]
- Etschmann, M.M.W.; Sell, D.; Schrader, J. Screening of Yeasts for the Production of the Aroma Compound 2-Phenylethanol in a Molasses-Based Medium. Biotechnol. Lett. 2003, 25, 531–536. [Google Scholar] [CrossRef]
- Chreptowicz, K.; Mierzejewska, J. Enhanced Bioproduction of 2-Phenylethanol in a Biphasic System with Rapeseed Oil. New Biotechnol. 2018, 42, 56–61. [Google Scholar] [CrossRef]
- Drężek, K.; Kozłowska, J.; Detman, A.; Mierzejewska, J. Development of a Continuous System for 2-Phenylethanol Bioproduction by Yeast on Whey Permeate-Based Medium. Molecules 2021, 26, 7388. [Google Scholar] [CrossRef]
- Carlquist, M.; Gibson, B.; Karagul Yuceer, Y.; Paraskevopoulou, A.; Sandell, M.; Angelov, A.I.; Gotcheva, V.; Angelov, A.D.; Etschmann, M.; de Billerbeck, G.M.; et al. Process Engineering for Bioflavour Production with Metabolically Active Yeasts—A Mini-Review. Yeast 2015, 32, 123–143. [Google Scholar] [CrossRef]
- Eltz, T.; Zimmermann, Y.; Haftmann, J.; Twele, R.; Francke, W.; Quezada-Euan, J.J.G.; Lunau, K. Enfleurage, Lipid Recycling and the Origin of Perfume Collection in Orchid Bees. Proc. R. Soc. B Biol. Sci. 2007, 274, 2843–2848. [Google Scholar] [CrossRef]
- Morrissey, J.P.; Etschmann, M.M.W.; Schrader, J.; de Billerbeck, G.M. Cell Factory Applications of the Yeast Kluyveromyces marxianus for the Biotechnological Production of Natural Flavour and Fragrance Molecules. Yeast 2015, 32, 3–16. [Google Scholar] [CrossRef]
- Gethins, L.; Guneser, O.; Demirkol, A.; Rea, M.C.; Stanton, C.; Ross, R.P.; Yuceer, Y.; Morrissey, J.P. Influence of Carbon and Nitrogen Source on Production of Volatile Fragrance and Flavour Metabolites by the Yeast Kluyveromyces marxianus. Yeast 2014, 32, 67–76. [Google Scholar] [CrossRef]
- Urit, T.; Manthey, R.; Bley, T.; Loser, C. Formation of Ethyl Acetate by Kluyveromyces marxianus on Whey: Influence of Aeration and Inhibition of Yeast Growth by Ethyl Acetate. Eng. Life Sci. 2013, 13, 247–260. [Google Scholar] [CrossRef]
- Löser, C.; Urit, T.; Stukert, A.; Bley, T. Formation of Ethyl Acetate from Whey by Kluyveromyces marxianus on a Pilot Scale. J. Biotechnol. 2013, 163, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, Y.; Yang, M.; Liu, Y.; Chen, K.; Long, C.; Deng, X. Mechanisms of Action for 2-Phenylethanol Isolated from Kloeckera Apiculata in Control of Penicillium Molds of Citrus Fruits. BMC Microbiol. 2014, 14, 242. [Google Scholar] [CrossRef] [PubMed]
- Garnier, L.; Penland, M.; Thierry, A.; Maillard, M.B.; Jardin, J.; Coton, M.; Leyva Salas, M.; Coton, E.; Valence, F.; Mounier, J. Antifungal Activity of Fermented Dairy Ingredients: Identification of Antifungal Compounds. Int. J. Food Microbiol. 2020, 322, 108574. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Purity, Antimicrobial Activities and Olfactory Evaluations of 2-Phenylethanol and Some Derivatives. J. Essent. Oil Res. 2008, 20, 82–85. [Google Scholar] [CrossRef]
- Urit, T.; Löser, C.; Wunderlich, M.; Bley, T. Formation of Ethyl Acetate by Kluyveromyces marxianus on Whey: Studies of the Ester Stripping. Bioprocess Biosyst. Eng. 2011, 34, 547–559. [Google Scholar] [CrossRef]
- Stark, D.; Zala, D.; Münch, T.; Sonnleitner, B.; Marison, I.W.; Von Stockar, U. Inhibition Aspects of the Bioconversion of L-Phenylalanine to 2-Phenylethanol by Saccharomyces Cerevisiae. Enzyme Microb. Technol. 2003, 32, 212–223. [Google Scholar] [CrossRef]
- Stark, D.; Münch, T.; Sonnleitner, B.; Marison, I.W.; von Stockar, U. Extractive Bioconversion of 2 Phenylethanol from L Phenylalanine by Saccharomyces Cerevisiae. Biotechnol. Prog. 2002, 18, 514–523. [Google Scholar] [CrossRef]
- Saini, P.; Beniwal, A.; Kokkiligadda, A.; Vij, S. Evolutionary Adaptation of Kluyveromyces marxianus Strain for Efficient Conversion of Whey Lactose to Bioethanol. Process Biochem. 2017, 62, 69–79. [Google Scholar] [CrossRef]
- Machado, J.J.B.; Coutinho, J.A.; Macedo, E.A. Solid–Liquid Equilibrium of α-Lactose in Ethanol/Water. Fluid Phase Equilib. 2000, 173, 121–134. [Google Scholar] [CrossRef]
- Okamoto, K.; Nakagawa, S.; Kanawaku, R.; Kawamura, S. Ethanol Production from Cheese Whey and Expired Milk by the Brown Rot Fungus Neolentinus lepideus. Fermentation 2019, 5, 49. [Google Scholar] [CrossRef]
- Sunarno, J.N.; Prasertsan, P.; Duangsuwan, W.; Kongjan, P.; Cheirsilp, B. Mathematical Modeling of Ethanol Production from Glycerol by Enterobacter Aerogenes Concerning the Influence of Impurities, Substrate, and Product Concentration. Biochem. Eng. J. 2020, 155, 107471. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G.; Aden, A.; Bozell, L.; Holladay, J.; White, J.; Manheim, A. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidated from Sugars and Synthesis Gas; US Department of Energy: Washington, DC, USA, 2004. [Google Scholar] [CrossRef]
- Gabardo, S.; Rech, R.; Rosa, C.A.; Ayub, M.A.Z. Dynamics of Ethanol Production from Whey and Whey Permeate Byimmobilized Strains of Kluyveromyces marxianus in Batch Andcontinuous Bioreactors. Renew. Energy 2014, 69, 89–96. [Google Scholar] [CrossRef]
- Parrondo, J.; Garcia, L.A.; Diaz, M. Production of an Alcoholic Beverage by Fermentation of Whey Permeate with Kluyveromyces Fragilis I: Primary Metabolism. J. Inst. Brew. 2000, 106, 367–375. [Google Scholar] [CrossRef]
- Christensen, A.D.; Kádár, Z.; Oleskowicz-Popiel, P.; Thomsen, M.H. Production of Bioethanol from Organic Whey Using Kluyveromyces marxianus. J. Ind. Microbiol. Biotechnol. 2011, 38, 283–289. [Google Scholar] [CrossRef]
- Gabardo, S.; Rech, R.; Ayub, M.A.Z. Performance of Different Immobilized-Cell Systems to Efficiently Produce Ethanol from Whey: Fluidized Batch, Packed-Bed and Fluidized Continuous Bioreactors. J. Chem. Technol. Biotechnol. 2012, 87, 1194–1201. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, J.; Xiao, D. Improved Ethanol Production by Mixed Immobilized Cells of Kluyveromyces marxianus and Saccharomyces Cerevisiae from Cheese Whey Powder Solution Fermentation. Appl. Biochem. Biotechnol. 2010, 160, 532–538. [Google Scholar] [CrossRef]
- Kargi, F.; Ozmihci, S. Utilization of Cheese Whey Powder (CWP) for Ethanol Fermentations: Effects of Operating Parameters. Enzyme Microb. Technol. 2006, 38, 711–718. [Google Scholar] [CrossRef]
- Grba, S.; Stehlik-Tomas, V.; Stanzer, D.; Vahcic, N.; Skrlin, A. Selection of Yeast Strain Kluyveromyces marxianus for Alcohol and Biomass Production on Whey. Chem. Biochem. Eng. Q. 2002, 16, 13–16. [Google Scholar]
- Rakonczás, N.; Kállai, Z.; Kovács, B.; Antal, G.; Szabó, S.; Holb, I.J. Comparison and Intercorrelation of Various Bentonite Products for Oenological Properties, Elemental Compositions, Volatile Compounds and Organoleptic Attributes of White Wine. Foods 2023, 12, 355. [Google Scholar] [CrossRef]
- Chreptowicz, K.; Wielechowska, M.; Główczyk-Zubek, J.; Rybak, E.; Mierzejewska, J. Production of Natural 2-Phenylethanol: From Biotransformation to Purified Product. Food Bioprod. Process. 2016, 100, 275–281. [Google Scholar] [CrossRef]









| Parameter | Variant Tested | |||
|---|---|---|---|---|
| Control | Rice Oil | Linseed Oil | Rapeseed Oil | |
| OD600 | 6.21 ± 1.23 | 12.10 ± 0.15 | 11.50 ± 0.75 | 9.66 ± 0.27 |
| DCW [g/L] | 4.62 ± 0.46 | 6.43 ± 0.53 | 8.08 ± 0.18 | 7.79 ± 0.75 |
| Lactose [g/L] | nd * | nd | nd | nd |
| L-phe [g/L] | 1.37 ± 0.17 | 0.99 ± 0.09 | 0.98 ± 0.03 | 0.96 ± 0.03 |
| 2-PEaq [g/L] | 2.45 ± 0.17 | 1.82 ± 0.07 | 1.71 ± 0.01 | 1.73 ± 0.05 |
| 2-PEorg [g/L] | - | 4.36 ± 0.01 | 4.10 ± 0.03 | 4.43 ± 0.18 |
| 2-PEtot [g/L] | 2.45 ± 0.17 | 2.33 ± 0.04 | 2.19 ± 0.02 | 2.27 ± 0.11 |
| Q [mg/L/h] | 51.04 ± 3.54 | 48.54 ± 0.83 | 45.63 ± 0.42 | 47.29 ± 2.29 |
| YP/S [g/g] | 0.67 | 0.58 | 0.54 | 0.56 |
| Parameter | Variant Tested | |
|---|---|---|
| 250 rpm | 500 rpm | |
| DCW [g/L] | 12.51 ± 2.72 | 11.35 ± 1.53 |
| Lactose [g/L] | nd * | nd |
| L-phe [g/L] | 1.25 ± 0.17 | 1.05 ± 0.09 |
| 2-PEaq [g/L] | 1.02 ± 0.17 | 1.44 ± 0.07 |
| 2-PEorg [g/L] | 2.84 ± 0.13 | 3.73 ± 0.01 |
| 2-PEtot [g/L] | 1.38 ± 0.03 | 1.90 ± 0.04 |
| Ethanol [g/L] | 3.62 ± 0.45 | nd |
| Q [mg/L/h] | 28.83 ± 0.65 | 39.54 ± 0.42 |
| YP/S [g/g] | 0.37 | 0.48 |
| Parameter | Variant Tested | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Shaking cultures (50 rpm) | ||||
| OD600 | 10.48 ± 0.52 | 8.82 ± 0.44 | 9.50 ± 0.48 | 9.07 ± 0.45 |
| DCW [g/L] | 13.83 ± 0.69 | 16.71 ± 0.84 | 21.17 ± 1.26 | 19.64 ± 2.96 |
| Lactose [g/L] | nd * | nd | nd | nd |
| Ethanol [g/L] | 69.63 ± 3.48 | 70.51 ± 3.53 | 73.52 ± 2.68 | 74.69 ± 0.87 |
| YP/X [g/g] | 5.03 | 4.22 | 3.47 | 3.80 |
| YP/S [g/g] | 0.50 | 0.50 | 0.53 | 0.53 |
| Glycerol [g/L] | 7.65 ± 0.65 | 8.08 ± 0.40 | 7.75 ± 0.49 | 7.65 ± 0.38 |
| Stationary cultures | ||||
| OD600 | 8.75 ± 0.86 | 8.83 ± 0.44 | 8.92 ± 0.48 | 9.33 ± 0.59 |
| DCW [g/L] | 17.33 ± 0.87 | 15.03 ± 1.77 | 16.41 ± 1.32 | 19.33 ± 1.63 |
| Lactose [g/L] | nd | nd | nd | nd |
| Ethanol [g/L] | 68.92 ± 3.45 | 71.77 ± 1.59 | 68.82 ± 2.44 | 65.40 ± 3.27 |
| YP/X [g/g] | 3.98 | 4.78 | 4.19 | 3.38 |
| YP/S [g/g] | 0.49 | 0.51 | 0.49 | 0.47 |
| Glycerol [g/L] | 7.62 ± 0.38 | 7.37 ± 0.37 | 7.71 ± 0.39 | 6.85 ± 0.34 |
| Medium | Composition |
|---|---|
| SAB | SAB 5 g/L meat peptone, 5 g/L casein peptone, 20 g/L glucose (Merck, Darmstadt, Germany), pH 5.6 |
| WP25 | 25 g/L whey permeate (Mlekpol, Grajewo, Poland), 5 g/L L-phe (Merck, Darmstadt, Germany), tap water, pH 5.4 |
| WP200 | 200 g/L whey permeate, tap water, pH 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drężek, K.; Sobczyk, M.K.; Kállai, Z.; Detman, A.; Bardadyn, P.; Mierzejewska, J. Valorisation of Whey Permeate in Sequential Bioprocesses towards Value-Added Products–Optimisation of Biphasic and Classical Batch Cultures of Kluyveromyces marxianus. Int. J. Mol. Sci. 2023, 24, 7560. https://doi.org/10.3390/ijms24087560
Drężek K, Sobczyk MK, Kállai Z, Detman A, Bardadyn P, Mierzejewska J. Valorisation of Whey Permeate in Sequential Bioprocesses towards Value-Added Products–Optimisation of Biphasic and Classical Batch Cultures of Kluyveromyces marxianus. International Journal of Molecular Sciences. 2023; 24(8):7560. https://doi.org/10.3390/ijms24087560
Chicago/Turabian StyleDrężek, Karolina, Maria Krystyna Sobczyk, Zoltán Kállai, Anna Detman, Paula Bardadyn, and Jolanta Mierzejewska. 2023. "Valorisation of Whey Permeate in Sequential Bioprocesses towards Value-Added Products–Optimisation of Biphasic and Classical Batch Cultures of Kluyveromyces marxianus" International Journal of Molecular Sciences 24, no. 8: 7560. https://doi.org/10.3390/ijms24087560
APA StyleDrężek, K., Sobczyk, M. K., Kállai, Z., Detman, A., Bardadyn, P., & Mierzejewska, J. (2023). Valorisation of Whey Permeate in Sequential Bioprocesses towards Value-Added Products–Optimisation of Biphasic and Classical Batch Cultures of Kluyveromyces marxianus. International Journal of Molecular Sciences, 24(8), 7560. https://doi.org/10.3390/ijms24087560