Hepatic Alterations in a BTBR T + Itpr3tf/J Mouse Model of Autism and Improvement Using Melatonin via Mitigation Oxidative Stress, Inflammation and Ferroptosis
Abstract
:1. Introduction
2. Results
2.1. Hepatic Histological Features


2.2. Liver Oxidative Stress, Inflammation and the Regulatory Pathways of Ferroptosis




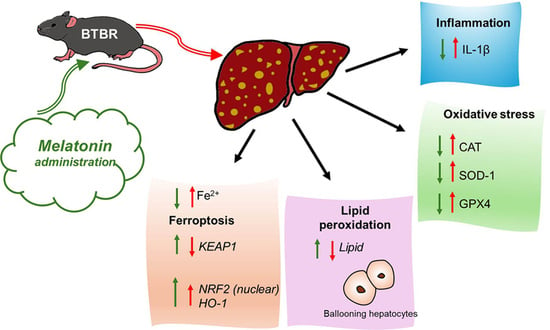
2.3. Melatonin Effects in the Liver of BTBR and CTR Animals
2.3.1. Histological Evaluation of Hepatic Tissue in BTBR after Melatonin Treatment


2.3.2. Oxidative Stress, Inflammation and Regulatory Pathways of Ferroptosis in BTBR Mice after Melatonin Treatment




3. Discussion
4. Materials and Methods
4.1. Experimental Groups
4.2. Sample Processing
4.3. Hepatic Morphological Evaluation
4.4. Perls Staining: Iron Accumulation
4.5. Immunohistochemical Evaluation
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, P.; Zhou, L.; Zhao, L.; Fei, X.; Wang, Z.; Liu, T.; Tang, Y.; Li, D.; Gong, H.; Luo, Y.; et al. Puerarin attenuates valproate-induced features of ASD in male mice via regulating Slc7a11-dependent ferroptosis. Neuropsychopharmacology 2023. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Gu, Y.; Zhang, J.; Hu, X.; Wu, H.; Yuan, T.; Zhao, D. A scoping review of physiological biomarkers in autism. Front. Neurosci. 2023, 17, 1269880. [Google Scholar] [CrossRef] [PubMed]
- Precenzano, F.; Ruberto, M.; Parisi, L.; Salerno, M.; Maltese, A.; Verde, D.; Tripi, G.; Romano, P.; Di Folco, A.; Di Filippo, T.; et al. Sleep habits in children affected by autism spectrum disorders: A preliminary case-control study. Acta Med. Mediter. 2017, 33, 405–409. [Google Scholar] [CrossRef]
- Sharma, A.; Mehan, S. Targeting PI3K-AKT/mTOR signaling in the prevention of autism. Neurochem. Int. 2021, 147, 105067. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef]
- Watts, T.J. The pathogenesis of autism. Clin. Med. Pathol. 2008, 1, 99–103. [Google Scholar] [CrossRef]
- Joon, P.; Kumar, A.; Parle, M. What is autism? Pharmacol. Rep. 2021, 73, 1255–1264. [Google Scholar] [CrossRef]
- Trinchese, G.; Cimmino, F.; Cavaliere, G.; Catapano, A.; Fogliano, C.; Lama, A.; Pirozzi, C.; Cristiano, C.; Russo, R.; Petrella, L.; et al. The Hepatic Mitochondrial alterations exacerbate meta-inflammation in autism spectrum disorders. Antioxidants 2022, 11, 1990. [Google Scholar] [CrossRef]
- Mirzavandi, F.; Sabet, N.; Aminzadeh, A.; Heidari, M.; Pouya, F.; Moslemizadeh, A.; Parizi, A.S.; Bashiri, H. Effects of varied-intensity endurance exercise training on oxidative and antioxidant factors in the liver of rats with valproic acid-induced autism. Acta Neurobiol. Exp. 2023, 83, 25–33. [Google Scholar] [CrossRef]
- Singh, R.; Kisku, A.; Kungumaraj, H.; Nagaraj, V.; Pal, A.; Kumar, S.; Sulakhiya, K. Autism spectrum disorders: A recent update on targeting inflammatory pathways with natural anti-inflammatory agents. Biomedicines 2023, 11, 115. [Google Scholar] [CrossRef]
- Messina, A.; Monda, V.; Sessa, F.; Valenzano, A.; Salerno, M.; Bitetti, I.; Precenzano, F.; Marotta, R.; Lavano, F.; Lavano, S.M.; et al. Sympathetic, metabolic adaptations, and oxidative stress in autism spectrum disorders: How far from physiology? Front. Physiol. 2018, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and oxidative stress in the pathogenesis of autism spectrum disorder. Int. J. Mol. Sci. 2023, 24, 5487. [Google Scholar] [CrossRef]
- Braun, J.M.; Kahn, R.S.; Froehlich, T.; Auinger, P.; Lanphear, B.P. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ. Health Perspect. 2006, 114, 1904–1909. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. A review of research trends in physiological abnormalities in autism spectrum disorders: Immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol. Psychiatry 2012, 17, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism. 2017, 8, 13. [Google Scholar] [CrossRef]
- Aschner, M.; Skalny, A.V.; Martins, A.C.; Sinitskii, A.I.; Farina, M.; Lu, R.; Barbosa, F., Jr.; Gluhcheva, Y.G.; Santamaria, A.; Tinkov, A.A. Ferroptosis as a mechanism of non-ferrous metal toxicity. Arch. Toxicol. 2022, 96, 2391–2417. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Belaidi, A.A.; Bush, A.I.; Ayton, S. Ferroptosis as a mechanism of neurodegeneration in Alzheimer’s disease. J. Neurochem. 2021, 159, 804–825. [Google Scholar] [CrossRef]
- Wu, H.; Luan, Y.; Wang, H.; Zhang, P.; Liu, S.; Wang, P.; Cao, Y.; Sun, H.; Wu, L. Selenium inhibits ferroptosis and ameliorates autistic-like behaviors of BTBR mice by regulating the Nrf2/GPx4 pathway. Brain Res. Bull. 2022, 183, 38–48. [Google Scholar] [CrossRef]
- McFarlane, H.G.; Kusek, G.K.; Yang, M.; Phoenix, J.L.; Bolivar, V.J.; Crawley, J.N. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008, 7, 152–163, Erratum in Genes Brain Behav. 2008, 7, 163. [Google Scholar] [CrossRef]
- Cao, C.; Wang, D.; Zou, M.; Sun, C.; Wu, L. Untargeted metabolomics reveals hepatic metabolic disorder in the BTBR mouse model of autism and the significant role of liver in autism. Cell Biochem. Funct. 2023, 41, 553–563. [Google Scholar] [CrossRef]
- Mortezaee, K.; Khanlarkhani, N. Melatonin application in targeting oxidative-induced liver injuries: A review. J. Cell Physiol. 2018, 233, 4015–4032. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.A.; Shaukat, A.; Wu, K.; Rajput, I.R.; Baloch, D.M.; Akhtar, R.W.; Raza, M.A.; Najda, A.; Rafał, P.; Albrakati, A.; et al. Luteolin alleviates aflatoxinB1-induced apoptosis and oxidative stress in the liver of mice through activation of Nrf2 signaling pathway. Antioxidants 2021, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Rezzani, R.; Franco, C. Liver, oxidative stress and metabolic syndromes. Nutrients 2021, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.M.; Shalaby, R.H.; Alabiad, M.A.; Abdelrahman, D.I.; Alorini, M.; Jaber, F.A.; Hassan, S.M.A. Evening primrose oil attenuates oxidative stress, inflammation, fibrosis, apoptosis, and ultrastructural alterations induced by metanil yellow in the liver of rat: A histological, immunohistochemical, and biochemical study. Ultrastruct. Pathol. 2023, 47, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.K.; Gopal, T.; Kalari Kandy, R.R.; Boopathy, L.K.; Perumal, S.K.; Ganesan, M.; Rasineni, K.; Donohue, T.M., Jr.; Osna, N.A.; Kharbanda, K.K. Mitochondrial Dysfunction-associated mechanisms in the development of chronic liver diseases. Biology 2023, 12, 1311. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, H.; Zou, M.; Li, L.; Li, Q.; Sun, C.; Xia, W.; Cao, Y.; Wu, L. Folic acid improves abnormal behavior via mitigation of oxidative stress, inflammation, and ferroptosis in the BTBR T+ tf/J mouse model of autism. J. Nutr. Biochem. 2019, 71, 98–109. [Google Scholar] [CrossRef]
- Granata, S.; Votrico, V.; Spadaccino, F.; Catalano, V.; Netti, G.S.; Ranieri, E.; Stallone, G.; Zaza, G. Oxidative stress and ischemia/reperfusion injury in kidney transplantation: Focus on ferroptosis, mitophagy and new antioxidants. Antioxidants 2022, 11, 769. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Tak, J.; Kim, S.G. Effects of toxicants on endoplasmic reticulum stress and hepatic cell fate determination. Toxicol. Res. 2023, 39, 533–547. [Google Scholar] [CrossRef]
- van Noorden, C.J.F.; Breznik, B.; Novak, M.; van Dijck, A.J.; Tanan, S.; Vittori, M.; Bogataj, U.; Bakker, N.; Khoury, J.D.; Molenaar, R.J.; et al. Cell biology meets cell metabolism: Energy production is similar in stem cells and in cancer stem cells in brain and bone marrow. J. Histochem. Cytochem. 2022, 70, 29–51. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Capelletti, M.M.; Manceau, H.; Puy, H.; Peoc’h, K. Ferroptosis in Liver Diseases: An Overview. Int. J. Mol. Sci. 2020, 21, 4908. [Google Scholar] [CrossRef] [PubMed]
- Korczowska-Łącka, I.; Słowikowski, B.; Piekut, T.; Hurła, M.; Banaszek, N.; Szymanowicz, O.; Jagodziński, P.P.; Kozubski, W.; Permoda-Pachuta, A.; Dorszewska, J. Disorders of endogenous and exogenous antioxidants in neurological diseases. Antioxidants 2023, 12, 1811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jia, X.; Lin, D.; Ma, J. Melatonin and ferroptosis: Mechanisms and therapeutic implications. Biochem. Pharmacol. 2023, 218, 115909. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Kang, J.W.; Hong, J.M.; Lee, S.M. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J. Pineal Res. 2016, 60, 383–393. [Google Scholar] [CrossRef]
- Zhang, J.J.; Meng, X.; Li, Y.; Zhou, Y.; Xu, D.P.; Li, S.; Li, H.B. Effects of melatonin on liver injuries and diseases. Int. J. Mol. Sci. 2017, 18, 673. [Google Scholar] [CrossRef]
- Miao, Z.; Miao, Z.; Teng, X.; Xu, S. Melatonin alleviates lead-induced fatty liver in the common carps (Cyprinus carpio) via gut-liver axis. Environ. Pollut. 2023, 317, 120730. [Google Scholar] [CrossRef]
- Gevezova, M.; Sbirkov, Y.; Sarafian, V.; Plaimas, K.; Suratanee, A.; Maes, M. Autistic spectrum disorder (ASD-Gene, molecular and pathway signatures linking systemic inflammation, mitochondrial dysfunction, transsynaptic signalling, and neurodevelopment. Brain Behav. Immun. Health 2023, 30, 100646. [Google Scholar] [CrossRef]
- Heinke, P.; Rost, F.; Rode, J.; Trus, P.; Simonova, I.; Lázár, E.; Feddema, J.; Welsch, T.; Alkass, K.; Salehpour, M.; et al. Diploid hepatocytes drive physiological liver renewal in adult humans. Cell Syst. 2022, 13, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Huttner, H.B.; Bergmann, O.; Salehpour, M.; Rácz, A.; Tatarishvili, J.; Lindgren, E.; Csonka, T.; Csiba, L.; Hortobágyi, T.; Méhes, G.; et al. The age and genomic integrity of neurons after cortical stroke in humans. Nat. Neurosci. 2014, 17, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of cell generation and turnover in the human heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, J.E.; Brégerie, O.; Robert, A.; Debey, P.; Brechot, C.; Desdouets, C. Liver cell polyploidization: A pivotal role for binuclear hepatocytes. J. Biol. Chem. 2003, 278, 19095–19101. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, J.; Kuchna, I.; Nowicki, K.; Imaki, H.; Wegiel, J.; Marchi, E.; Ma, S.Y.; Chauhan, A.; Chauhan, V.; Bobrowicz, T.W.; et al. The neuropathology of autism: Defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 2010, 119, 755–770. [Google Scholar] [CrossRef]
- Calabrese, V.; Giordano, J.; Ruggieri, M.; Berritta, D.; Trovato, A.; Ontario, M.L.; Bianchini, R.; Calabrese, E.J. Hormesis, cellular stress response, and redox homeostasis in autism spectrum disorders. J. Neurosci. Res. 2016, 94, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, C.; Carbone, A.; Panelli, P.; Giambra, V.; Bossi, M.; Mazzoccoli, G.; De Filippis, L. Neural stem cells from Shank3-ko mouse model autism spectrum disorders. Mol. Neurobiol. 2020, 57, 1502–1515. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative stress in autism spectrum disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V.; Brown, W.T.; Cohen, I. Oxidative stress in autism: Increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin--the antioxidant proteins. Life Sci. 2004, 75, 2539–2549. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Cui, S.; Ghai, A.; Deng, Y.; Li, S.; Zhang, R.; Egbulefu, C.; Liang, G.; Achilefu, S.; Ye, J. Identification of hyperoxidized PRDX3 as a ferroptosis marker reveals ferroptotic damage in chronic liver diseases. Mol. Cell 2023, 83, 3931–3939.e5. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013, 34, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fu, X.; Liao, X.; Li, Y. Nrf2 activators as dietary phytochemicals against oxidative stress, inflammation, and mitochondrial dysfunction in autism spectrum disorders: A systematic review. Front. Psychiatry 2020, 11, 561998. [Google Scholar] [CrossRef]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef]
- Borsani, E.; Bonomini, F.; Bonini, S.A.; Premoli, M.; Maccarinelli, G.; Giugno, L.; Mastinu, A.; Aria, F.; Memo, M.; Rezzani, R. Role of melatonin in autism spectrum disorders in a male murine transgenic model: Study in the prefrontal cortex. J. Neurosci. Res. 2022, 100, 780–797. [Google Scholar] [CrossRef]
- Franco, C.; Bonomini, F.; Borsani, E.; Castrezzati, S.; Franceschetti, L.; Rezzani, R. Involvement of intestinal goblet cells and changes in sodium glucose transporters expression: Possible therapeutic targets in autistic BTBR T+Itpr3tf/J mice. Int. J. Environ. Res. Public Health 2021, 18, 11328. [Google Scholar] [CrossRef]
- Rezzani, R.; Franco, C.; Favero, G.; Rodella, L.F. Ghrelin-mediated pathway in Apolipoprotein-E deficient mice: A survival system. Am. J. Transl. Res. 2019, 11, 4263–4276. [Google Scholar]
- Perls, M. Nachweis von Eisenoxyd in geweissen Pigmentation. In Virchows Archiv für Pathologische Anatomie und Physiologie und für Klinische Medizin; Springer Publishing: New York, NY, USA, 1867; Volume 39, p. 42. [Google Scholar]
- Bancroft, G.J.; Gamble, M. Theory and Practices of Histological Techniques; Churchill Livingstone Elsevier: Philadelphia, PA, USA, 2008. [Google Scholar]
- Alwahaibi, N.Y.; Alkhatri, A.S.; Kumar, J.S. Hematoxylin and eosin stain shows a high sensitivity but sub-optimal specificity in demonstrating iron pigment in liver biopsies. Int. J. Appl. Basic Med. Res. 2015, 5, 169–171. [Google Scholar] [CrossRef]
- Rezzani, R.; Rodella, L.F.; Bonomini, F.; Tengattini, S.; Bianchi, R.; Reiter, R.J. Beneficial effects of melatonin in protecting against cyclosporine A-induced cardiotoxicity are receptor mediated. J. Pineal Res. 2006, 41, 288–295. [Google Scholar] [CrossRef]
- Franco, C.; Gianò, M.; Favero, G.; Rezzani, R. Impairment in the intestinal morphology and in the immunopositivity of toll-like receptor-4 and other proteins in an autistic mouse model. Int. J. Mol. Sci. 2022, 23, 8731. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Favero, G.; Rodella, L.F.; Moghadasian, M.H.; Rezzani, R. Melatonin modulation of sirtuin-1 attenuates liver injury in a hypercholesterolemic mouse model. BioMed Res. Int. 2018, 2018, 7968452. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, A.; Favero, G.; Lavazza, A.; Golic, I.; Aleksic, M.; Korac, A.; Rodella, L.F.; Rezzani, R. Hepatic macrosteatosis is partially converted to microsteatosis by melatonin supplementation in ob/ob mice non-alcoholic fatty liver disease. PLoS ONE 2016, 11, e0148115. [Google Scholar] [CrossRef] [PubMed]
- Chieco, P.; Jonker, A.; De Boer, B.A.; Ruijter, J.M.; Van Noorden, C.J. Image cytometry: Protocols for 2D and 3D quantification in microscopic images. Prog. Histochem. Cytochem. 2013, 47, 211–333. [Google Scholar] [CrossRef]
- Wiseman, E.J.; Moss, J.I.; Atkinson, J.; Baakza, H.; Hayes, E.; Willis, S.E.; Waring, P.M.; Rodriguez Canales, J.; Jones, G.N. Epitope lability of phosphorylated biomarkers of the DNA Damage response pathway results in increased vulnerability to effects of delayed or incomplete formalin fixation. J. Histochem. Cytochem. 2023, 71, 237–257. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezzani, R.; Gianò, M.; Pinto, D.; Rinaldi, F.; van Noorden, C.J.F.; Favero, G. Hepatic Alterations in a BTBR T + Itpr3tf/J Mouse Model of Autism and Improvement Using Melatonin via Mitigation Oxidative Stress, Inflammation and Ferroptosis. Int. J. Mol. Sci. 2024, 25, 1086. https://doi.org/10.3390/ijms25021086
Rezzani R, Gianò M, Pinto D, Rinaldi F, van Noorden CJF, Favero G. Hepatic Alterations in a BTBR T + Itpr3tf/J Mouse Model of Autism and Improvement Using Melatonin via Mitigation Oxidative Stress, Inflammation and Ferroptosis. International Journal of Molecular Sciences. 2024; 25(2):1086. https://doi.org/10.3390/ijms25021086
Chicago/Turabian StyleRezzani, Rita, Marzia Gianò, Daniela Pinto, Fabio Rinaldi, Cornelis J. F. van Noorden, and Gaia Favero. 2024. "Hepatic Alterations in a BTBR T + Itpr3tf/J Mouse Model of Autism and Improvement Using Melatonin via Mitigation Oxidative Stress, Inflammation and Ferroptosis" International Journal of Molecular Sciences 25, no. 2: 1086. https://doi.org/10.3390/ijms25021086
APA StyleRezzani, R., Gianò, M., Pinto, D., Rinaldi, F., van Noorden, C. J. F., & Favero, G. (2024). Hepatic Alterations in a BTBR T + Itpr3tf/J Mouse Model of Autism and Improvement Using Melatonin via Mitigation Oxidative Stress, Inflammation and Ferroptosis. International Journal of Molecular Sciences, 25(2), 1086. https://doi.org/10.3390/ijms25021086