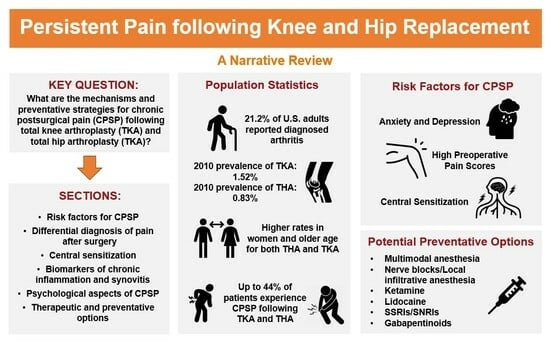
Mechanisms and Preventative Strategies for Persistent Pain following Knee and Hip Joint Replacement Surgery: A Narrative Review
Abstract
:1. Introduction
2. Risk Factors for CPSP Following TKA and THA
3. Potential Mechanisms of CPSP
3.1. Differential Diagnosis of Pain after Surgery
3.2. Central Sensitization
3.3. Chronic Inflammation and Synovitis
3.4. Other Biomarkers/Mechanisms
3.5. Psychological Aspects of Persistent Postoperative Pain
4. Potential Preventative Options
4.1. Multimodal Pharmacological Analgesia
4.2. Regional Anesthesia
4.3. Ketamine
4.4. Lidocaine
4.5. SSRIs and SNRIs
4.6. Gabapentinoids
| Intervention | Support for Prevention | Summary of Evidence |
|---|---|---|
| Multimodal pharmacological analgesia | Theoretical (unexplored in THA/TKA) | Postoperative pain is associated with CPSP following TKA/THA [19,22,23,24,25]. Multimodal analgesia is a leading strategy for reduction of postoperative pain [68,71,72,73,74,75]. |
| Regional Anesthesia | Mixed, largely negative | Of 11 nerve block and LIA studies identified, 1 showed favorable evidence. Ten showed no difference [84]. |
| Ketamine | Mixed | Two RCTs showed reduction in CPSP at 6 months following perioperative ketamine [94,95]. One RCT showed no difference at 6 or 12 months [96]. |
| Lidocaine | Positive in other surgeries, unexplored following THA/TKA | One meta-analysis w/12 RCTs found reduction in CPSP at <6 months. Two meta-analyses (6 RCTs and 4 RCTs) saw reduction at >3 months. |
| SSRIs and SNRIs | Negative in THA/TKA, positive in other surgeries | Two RCTs w/negative results following THA/TKA [103,104]. Three meta-analyses in other surgeries w/positive results [102,105,106]. |
| Gabapentinoids | Mixed | One RCT of 240 patients found reduction in CPSP at 6 months [109]. Another RCT of 60 patients found no significant effect [110]. |
5. Therapeutic Options
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fallon, E.A. Prevalence of Diagnosed Arthritis—United States, 2019–2021. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- DeRogatis, M.; Anis, H.K.; Sodhi, N.; Ehiorobo, J.O.; Chughtai, M.; Bhave, A.; Mont, M.A. Non-Operative Treatment Options for Knee Osteoarthritis. Ann. Transl. Med. 2019, 7, S245. [Google Scholar] [CrossRef]
- Neuprez, A.; Neuprez, A.H.; Kaux, J.-F.; Kurth, W.; Daniel, C.; Thirion, T.; Huskin, J.-P.; Gillet, P.; Bruyère, O.; Reginster, J.-Y. Total Joint Replacement Improves Pain, Functional Quality of Life, and Health Utilities in Patients with Late-Stage Knee and Hip Osteoarthritis for up to 5 Years. Clin. Rheumatol. 2020, 39, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Shichman, I.; Roof, M.; Askew, N.; Nherera, L.; Rozell, J.C.; Seyler, T.M.; Schwarzkopf, R. Projections and Epidemiology of Primary Hip and Knee Arthroplasty in Medicare Patients to 2040–2060. JBJS Open Access 2023, 8, e22.00112. [Google Scholar] [CrossRef] [PubMed]
- Kremers, H.M.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Joint Surg. Am. 2015, 97, 1386. [Google Scholar] [CrossRef] [PubMed]
- Inacio, M.C.S.; Paxton, E.W.; Graves, S.E.; Namba, R.S.; Nemes, S. Projected Increase in Total Knee Arthroplasty in the United States—An Alternative Projection Model. Osteoarthr. Cartil. 2017, 25, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A. Epidemiology of Knee and Hip Arthroplasty: A Systematic Review. Open Orthop. J. 2011, 5, 80–85. [Google Scholar] [CrossRef]
- Liu, W.Y.; van der Steen, M.C.; van Wensen, R.J.A.; van Kempen, R.W.T.M. Recovery Patterns in Patients Undergoing Revision Surgery of the Primary Knee Prosthesis. J. Exp. Orthop. 2021, 8, 117. [Google Scholar] [CrossRef]
- van der Wees, P.J.; Wammes, J.J.G.; Akkermans, R.P.; Koetsenruijter, J.; Westert, G.P.; van Kampen, A.; Hannink, G.; de Waal-Malefijt, M.; Schreurs, B.W. Patient-Reported Health Outcomes after Total Hip and Knee Surgery in a Dutch University Hospital Setting: Results of Twenty Years Clinical Registry. BMC Musculoskelet. Disord. 2017, 18, 97. [Google Scholar] [CrossRef]
- Tang, S.; Jin, Y.; Hou, Y.; Wang, W.; Zhang, J.; Zhu, W.; Zhang, W.; Gu, X.; Ma, Z. Predictors of Chronic Pain in Elderly Patients Undergoing Total Knee and Hip Arthroplasty: A Prospective Observational Study. J. Arthroplast. 2023, 38, 1693–1699. [Google Scholar] [CrossRef]
- Werner, M.U.; Kongsgaard, U.E. I. Defining Persistent Post-Surgical Pain: Is an Update Required? Br. J. Anaesth. 2014, 113, 1–4. [Google Scholar] [CrossRef] [PubMed]
- McNicol, E.D.; Schumann, R.; Haroutounian, S. A Systematic Review and Meta-Analysis of Ketamine for the Prevention of Persistent Post-Surgical Pain. Acta Anaesthesiol. Scand. 2014, 58, 1199–1213. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Santone, D.; Takahashi, M.; Dessouki, O.; Mahomed, N.N. Inflammatory Predictors of Ongoing Pain 2 Years Following Knee Replacement Surgery. Knee 2013, 20, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Wylde, V.; Beswick, A.; Bruce, J.; Blom, A.; Howells, N.; Gooberman-Hill, R. Chronic Pain after Total Knee Arthroplasty. EFORT Open Rev. 2018, 3, 461–470. [Google Scholar] [CrossRef]
- Ghoshal, A.; Bhanvadia, S.; Singh, S.; Yaeger, L.; Haroutounian, S. Factors Associated with Persistent Postsurgical Pain after Total Knee or Hip Joint Replacement: A Systematic Review and Meta-Analysis. Pain Rep. 2023, 8, e1052. [Google Scholar] [CrossRef] [PubMed]
- Rajamäki, T.J.; Jämsen, E.; Puolakka, P.A.; Nevalainen, P.I.; Moilanen, T. Diabetes Is Associated with Persistent Pain after Hip and Knee Replacement. Acta Orthop. 2015, 86, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.N.; Rice, D.A.; McNair, P.J.; Kluger, M. Predictors of Persistent Pain after Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. Br. J. Anaesth. 2015, 114, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Varallo, G.; Giusti, E.M.; Manna, C.; Castelnuovo, G.; Pizza, F.; Franceschini, C.; Plazzi, G. Sleep Disturbances and Sleep Disorders as Risk Factors for Chronic Postsurgical Pain: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2022, 63, 101630. [Google Scholar] [CrossRef] [PubMed]
- Nikolajsen, L.; Brandsborg, B.; Lucht, U.; Jensen, T.S.; Kehlet, H. Chronic Pain Following Total Hip Arthroplasty: A Nationwide Questionnaire Study. Acta Anaesthesiol. Scand. 2006, 50, 495–500. [Google Scholar] [CrossRef]
- Erlenwein, J.; Müller, M.; Falla, D.; Przemeck, M.; Pfingsten, M.; Budde, S.; Quintel, M.; Petzke, F. Clinical Relevance of Persistent Postoperative Pain after Total Hip Replacement—A Prospective Observational Cohort Study. J. Pain Res. 2017, 10, 2183–2193. [Google Scholar] [CrossRef]
- Beswick, A.D.; Wylde, V.; Gooberman-Hill, R.; Blom, A.; Dieppe, P. What Proportion of Patients Report Long-Term Pain after Total Hip or Knee Replacement for Osteoarthritis? A Systematic Review of Prospective Studies in Unselected Patients. BMJ Open 2012, 2, e000435. [Google Scholar] [CrossRef] [PubMed]
- Puolakka, P.A.E.; Rorarius, M.G.F.; Roviola, M.; Puolakka, T.J.S.; Nordhausen, K.; Lindgren, L. Persistent Pain Following Knee Arthroplasty. Eur. J. Anaesthesiol. 2010, 27, 455–460. [Google Scholar] [CrossRef]
- Lloret-Linares, D.C. Predictive Factors of Chronic Post-Surgical Pain At6 Months Following Knee Replacement: Influenceof Postoperative Pain Trajectory and Genetics. Pain Physician 2016, 5, E729–E741. [Google Scholar] [CrossRef]
- Tian, M.; Li, Z.; Chen, X.; Wu, Q.; Shi, H.; Zhu, Y.; Shi, Y. Prevalence and Predictors of Chronic Pain with Two-Year Follow-Up after Knee Arthroplasty. J. Pain Res. 2022, 15, 1091–1105. [Google Scholar] [CrossRef]
- Lavand’homme, P.M.; Grosu, I.; France, M.-N.; Thienpont, E. Pain Trajectories Identify Patients at Risk of Persistent Pain after Knee Arthroplasty: An Observational Study. Clin. Orthop. 2014, 472, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Chodór, P.; Kruczyński, J. Predicting Persistent Unclear Pain Following Primary Total Knee Arthroplasty. Ortop. Traumatol. Rehabil. 2016, 18, 527–536. [Google Scholar] [CrossRef]
- Wright, A.; Moss, P.; Sloan, K.; Beaver, R.J.; Pedersen, J.B.; Vehof, G.; Borge, H.; Maestroni, L.; Cheong, P. Abnormal Quantitative Sensory Testing Is Associated with Persistent Pain One Year after TKA. Clin. Orthop. 2015, 473, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.K.; Simonsen, O.; Laursen, M.B.; Nielsen, T.A.; Rasmussen, S.; Arendt-Nielsen, L. Chronic Postoperative Pain after Primary and Revision Total Knee Arthroplasty. Clin. J. Pain 2015, 31, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-A.; Song, E.-K.; Seon, J.-K.; Park, K.-S.; Shin, Y.-J.; Yang, H.-Y. Causes of Aseptic Persistent Pain after Total Knee Arthroplasty. Clin. Orthop. Surg. 2017, 9, 50–56. [Google Scholar] [CrossRef]
- Anil, U.; Singh, V.; Schwarzkopf, R. Diagnosis and Detection of Subtle Aseptic Loosening in Total Hip Arthroplasty. J. Arthroplast. 2022, 37, 1494–1500. [Google Scholar] [CrossRef]
- Roman, M.D.; Russu, O.; Mohor, C.; Necula, R.; Boicean, A.; Todor, A.; Mohor, C.; Fleaca, S.R. Outcomes in Revision Total Knee Arthroplasty (Review). Exp. Ther. Med. 2022, 23, 29. [Google Scholar] [CrossRef]
- Senthi, S.; Munro, J.T.; Pitto, R.P. Infection in Total Hip Replacement: Meta-Analysis. Int. Orthop. 2011, 35, 253–260. [Google Scholar] [CrossRef]
- Kerver, A.L.A.; Leliveld, M.S.; den Hartog, D.; Verhofstad, M.H.J.; Kleinrensink, G.J. The Surgical Anatomy of the Infrapatellar Branch of the Saphenous Nerve in Relation to Incisions for Anteromedial Knee Surgery. J. Bone Jt. Surg. 2013, 95, 2119–2125. [Google Scholar] [CrossRef]
- Nahabedian, M.Y.; Johnson, C.A. Operative Management of Neuromatous Knee Pain: Patient Selection and Outcome. Ann. Plast. Surg. 2001, 46, 15. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, A.; Valentino, L.; Giovanni Mazzoleni, M.; Tarantino, A.; Calvisi, V. Painful Total Knee Arthroplasty: Infrapatellar Branch of the Saphenous Nerve Selective Denervation. A Case Series. Knee 2022, 39, 197–202. [Google Scholar] [CrossRef] [PubMed]
- James, N.F.; Kumar, A.R.; Wilke, B.K.; Shi, G.G. Incidence of Encountering the Infrapatellar Nerve Branch of the Saphenous Nerve During a Midline Approach for Total Knee Arthroplasty. JAAOS Glob. Res. Rev. 2019, 3, e19.00160. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.-M.; Meister, D.W.; Graner, K.C.; Ninomiya, J.T. Selective Denervation for Persistent Knee Pain after Total Knee Arthroplasty: A Report of 50 Cases. J. Arthroplast. 2017, 32, 968–973. [Google Scholar] [CrossRef]
- Preston, S.; Petrera, M.; Kim, C.; Zywiel, M.G.; Gandhi, R. Towards an Understanding of the Painful Total Knee: What Is the Role of Patient Biology? Curr. Rev. Musculoskelet. Med. 2016, 9, 388–395. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J. Pain Off. J. Am. Pain Soc. 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Schwartzman, R.J.; Grothusen, J.; Kiefer, T.R.; Rohr, P. Neuropathic Central Pain: Epidemiology, Etiology, and Treatment Options. Arch. Neurol. 2001, 58, 1547–1550. [Google Scholar] [CrossRef]
- Mendell, L.M. The Path to Discovery of Windup and Central Sensitization. Front. Pain Res. 2022, 3, 833104. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.J.; Kang, K.H.; Kim, M.J.; In, Y. Diagnosis of Central Sensitization and Its Effects on Postoperative Outcomes Following Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Wylde, V.; Sayers, A.; Lenguerrand, E.; Gooberman-Hill, R.; Pyke, M.; Beswick, A.D.; Dieppe, P.; Blom, A.W. Preoperative Widespread Pain Sensitization and Chronic Pain after Hip and Knee Replacement: A Cohort Analysis. Pain 2015, 156, 47–54. [Google Scholar] [CrossRef]
- Koh, I.J.; Kang, B.M.; Kim, M.S.; Choi, K.Y.; Sohn, S.; In, Y. How Does Preoperative Central Sensitization Affect Quality of Life Following Total Knee Arthroplasty? J. Arthroplast. 2020, 35, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, E.; Kasai, T.; Araki, R.; Sasaki, T.; Wakai, Y.; Akaishi, K.; Chiba, D.; Kimura, Y.; Yamamoto, Y.; Tsuda, E.; et al. Central Sensitization and Postoperative Improvement of Quality of Life in Total Knee and Total Hip Arthroplasty: A Prospective Observational Study. Prog. Rehabil. Med. 2022, 7, 20220009. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Koh, I.J.; Sohn, S.; Kang, B.M.; Kwak, D.H.; In, Y. Central Sensitization Is a Risk Factor for Persistent Postoperative Pain and Dissatisfaction in Patients Undergoing Revision Total Knee Arthroplasty. J. Arthroplast. 2019, 34, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Imagama, T.; Okazaki, T.; Matsuki, Y.; Kaneoka, T.; Kawakami, T.; Yamazaki, K.; Sakai, T. Negative Correlation between Central Sensitization and Forgotten Joint Score-12 after Total Hip Arthroplasty. J. Orthop. Surg. 2023, 18, 691. [Google Scholar] [CrossRef]
- Wylde, V.; Palmer, S.; Learmonth, I.D.; Dieppe, P. The Association between Pre-Operative Pain Sensitisation and Chronic Pain after Knee Replacement: An Exploratory Study. Osteoarthr. Cartil. 2013, 21, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Kurien, T.; Kerslake, R.W.; Graven-Nielsen, T.; Arendt-Nielsen, L.; Auer, D.P.; Edwards, K.; Scammell, B.E.; Petersen, K.K.-S. Chronic Postoperative Pain after Total Knee Arthroplasty: The Potential Contributions of Synovitis, Pain Sensitization and Pain Catastrophizing—An Explorative Study. Eur. J. Pain Lond. Engl. 2022, 26, 1979–1989. [Google Scholar] [CrossRef]
- Maquet, D.; Croisier, J.-L.; Demoulin, C.; Crielaard, J.-M. Pressure Pain Thresholds of Tender Point Sites in Patients with Fibromyalgia and in Healthy Controls. Eur. J. Pain Lond. Engl. 2004, 8, 111–117. [Google Scholar] [CrossRef]
- Cheng, J.C.; Erpelding, N.; Kucyi, A.; DeSouza, D.D.; Davis, K.D. Individual Differences in Temporal Summation of Pain Reflect Pronociceptive and Antinociceptive Brain Structure and Function. J. Neurosci. 2015, 35, 9689–9700. [Google Scholar] [CrossRef]
- Dainese, P.; Mahieu, H.; De Mits, S.; Wittoek, R.; Stautemas, J.; Calders, P. Associations between Markers of Inflammation and Altered Pain Perception Mechanisms in People with Knee Osteoarthritis: A Systematic Review. RMD Open 2023, 9, e002945. [Google Scholar] [CrossRef] [PubMed]
- Skrejborg, P.; Petersen, K.K.; Kold, S.; Kappel, A.; Pedersen, C.; Østgaard, S.E.; Simonsen, O.; Arendt-Nielsen, L. Patients With High Chronic Postoperative Knee Pain 5 Years After Total Knee Replacement Demonstrate Low-Grad Inflammation, Impairment of Function, and High Levels of Pain Catastrophizing. Clin. J. Pain 2021, 37, 161. [Google Scholar] [CrossRef] [PubMed]
- Sideris, A.; Malahias, M.-A.; Birch, G.; Zhong, H.; Rotundo, V.; Like, B.J.; Otero, M.; Sculco, P.K.; Kirksey, M. Identification of Biological Risk Factors for Persistent Postoperative Pain after Total Knee Arthroplasty. Reg. Anesth. Pain Med. 2022, 47, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Noorbaloochi, S.; Knutson, K.L. Cytokine and Neuropeptide Levels Are Associated with Pain Relief in Patients with Chronically Painful Total Knee Arthroplasty: A Pilot Study. BMC Musculoskelet. Disord. 2017, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Mrazek, F.; Petrek, M. Variation in Cytokine Genes Can Contribute to Severity of Acetabular Osteolysis and Risk for Revision in Patients with ABG 1 Total Hip Arthroplasty: A Genetic Association Study. BMC Med. Genet. 2009, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; Wager, T.D.; Sutherland, S.P.; Labosky, P.A.; Balach, T.; Bayman, E.O.; Berardi, G.; Brummett, C.M.; Burns, J.; Buvanendran, A.; et al. Predicting Chronic Postsurgical Pain: Current Evidence and a Novel Program to Develop Predictive Biomarker Signatures. Pain 2023, 164, 1912–1926. [Google Scholar] [CrossRef] [PubMed]
- Bruehl, S.; Denton, J.S.; Lonergan, D.; Koran, M.E.; Chont, M.; Sobey, C.; Fernando, S.; Bush, W.S.; Mishra, P.; Thornton-Wells, T.A. Associations between KCNJ6 (GIRK2) Gene Polymorphisms and Pain-Related Phenotypes. Pain 2013, 154, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Costello, C.A.; Hu, T.; Liu, M.; Zhang, W.; Furey, A.; Fan, Z.; Rahman, P.; Randell, E.W.; Zhai, G. Metabolomics Signature for Non-Responders to Total Joint Replacement Surgery in Primary Osteoarthritis Patients: The Newfoundland Osteoarthritis Study. J. Orthop. Res. 2020, 38, 793–802. [Google Scholar] [CrossRef]
- Feeney, S.L. The Relationship between Pain and Negative Affect in Older Adults: Anxiety as a Predictor of Pain. J. Anxiety Disord. 2004, 18, 733–744. [Google Scholar] [CrossRef]
- Vissers, M.M.; Bussmann, J.B.; Verhaar, J.A.N.; Busschbach, J.J.V.; Bierma-Zeinstra, S.M.A.; Reijman, M. Psychological Factors Affecting the Outcome of Total Hip and Knee Arthroplasty: A Systematic Review. Semin. Arthritis Rheum. 2012, 41, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Masselin-Dubois, A.; Attal, N.; Fletcher, D.; Jayr, C.; Albi, A.; Fermanian, J.; Bouhassira, D.; Baudic, S. Are Psychological Predictors of Chronic Postsurgical Pain Dependent on the Surgical Model? A Comparison of Total Knee Arthroplasty and Breast Surgery for Cancer. J. Pain 2013, 14, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, M.T.; Testa, E.; Amsler, F.; Friederich, N.F. The Unhappy Total Knee Arthroplasty (TKA) Patient: Higher WOMAC and Lower KSS in Depressed Patients Prior and after TKA. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs-Rocker, A.; Schulz, K.; Järvinen, I.; Lefering, R.; Simanski, C.; Neugebauer, E.A.M. Psychosocial Predictors and Correlates for Chronic Post-Surgical Pain (CPSP)—A Systematic Review. Eur. J. Pain 2009, 13, 719–730. [Google Scholar] [CrossRef]
- Giusti, E.M.; Lacerenza, M.; Manzoni, G.M.; Castelnuovo, G. Psychological and Psychosocial Predictors of Chronic Postsurgical Pain: A Systematic Review and Meta-Analysis. Pain 2021, 162, 10. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.L.; Keefe, F.J.; Ang, D.C.; Slover, J.; Jensen, M.P.; Bair, M.J.; Kroenke, K.; Perera, R.A.; Reed, S.D.; McKee, D.; et al. Pain Coping Skills Training for Patients Who Catastrophize About Pain Prior to Knee Arthroplasty. J. Bone Joint Surg. Am. 2019, 101, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.M.-L.; Chan, S.W.-C.; Chair, S.-Y. Effectiveness of an Educational Intervention on Levels of Pain, Anxiety and Self-Efficacy for Patients with Musculoskeletal Trauma. J. Adv. Nurs. 2010, 66, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J. Poorly Controlled Postoperative Pain: Prevalence, Consequences, and Prevention. J. Pain Res. 2017, 10, 2287–2298. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent Postsurgical Pain: Risk Factors and Prevention. Lancet Lond. Engl. 2006, 367, 1618–1625. [Google Scholar] [CrossRef]
- Veal, F.C.; Bereznicki, L.R.E.; Thompson, A.J.; Peterson, G.M.; Orlikowski, C. Subacute Pain as a Predictor of Long-Term Pain Following Orthopedic Surgery. Medicine 2015, 94, e1498. [Google Scholar] [CrossRef]
- Soetjahjo, B.; Nefihancoro, U.H.; Ermawan, R.; Saputra, R.D.; Pranandaru, H. Postoperative Pain after Total Joint Arthroplasty: Pathophysiology and Current Pharmacological Pain Management. Biomol. Health Sci. J. 2022, 5, 129. [Google Scholar] [CrossRef]
- Wick, E.C.; Grant, M.C.; Wu, C.L. Postoperative Multimodal Analgesia Pain Management with Nonopioid Analgesics and Techniques: A Review. JAMA Surg. 2017, 152, 691–697. [Google Scholar] [CrossRef]
- Keohane, D.; Sheridan, G.; Harty, J. Perioperative Steroid Administration Improves Knee Function and Reduces Opioid Consumption in Bilateral Total Knee Arthroplasty. J. Orthop. 2020, 22, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Soffin, E.M.; Wu, C.L. Regional and Multimodal Analgesia to Reduce Opioid Use after Total Joint Arthroplasty: A Narrative Review. HSS J. Musculoskelet. J. Hosp. Spec. Surg. 2019, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Zajonz, D.; Fakler, J.K.M.; Dahse, A.-J.; Zhao, F.J.; Edel, M.; Josten, C.; Roth, A. Evaluation of a Multimodal Pain Therapy Concept for Chronic Pain after Total Knee Arthroplasty: A Pilot Study in 21 Patients. Patient Saf. Surg. 2017, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, H.; Zheng, C.; Wang, B.; Shen, P.; Xie, Z.; Qu, Y. The Efficacy of Pre-Emptive Analgesia on Pain Management in Total Knee Arthroplasty: A Mini-Review. Arthroplasty 2019, 1, 10. [Google Scholar] [CrossRef]
- McDonald, D.A.; Deakin, A.H.; Ellis, B.M.; Robb, Y.; Howe, T.E.; Kinninmonth, A.W.G.; Scott, N.B. The Technique of Delivery of Peri-Operative Analgesia Does Not Affect the Rehabilitation or Outcomes Following Total Knee Arthroplasty. Bone Jt. J. 2016, 98-B, 1189–1196. [Google Scholar] [CrossRef]
- Sean, V.W.T.; Chin, P.L.; Chia, S.L.; Yang, K.Y.; Lo, N.N.; Yeo, S.J. Single-Dose Periarticular Steroid Infiltration for Pain Management in Total Knee Arthroplasty: A Prospective, Double-Blind, Randomised Controlled Trial. Singap. Med. J. 2011, 52, 19–23. [Google Scholar]
- Motififard, M.; Omidian, A.; Badiei, S. Pre-Emptive Injection of Peri-Articular-Multimodal Drug for Post-Operative Pain Management in Total Knee Arthroplasty: A Double-Blind Randomized Clinical Trial. Int. Orthop. 2017, 41, 939–947. [Google Scholar] [CrossRef]
- Niemeläinen, M.; Kalliovalkama, J.; Aho, A.J.; Moilanen, T.; Eskelinen, A. Single Periarticular Local Infiltration Analgesia Reduces Opiate Consumption until 48 Hours after Total Knee Arthroplasty. Acta Orthop. 2014, 85, 614–619. [Google Scholar] [CrossRef]
- Williams, D.; Petruccelli, D.; Paul, J.; Piccirillo, L.; Winemaker, M.; de Beer, J. Continuous Infusion of Bupivacaine Following Total Knee Arthroplasty: A Randomized Control Trial Pilot Study. J. Arthroplast. 2013, 28, 479–484. [Google Scholar] [CrossRef]
- Wylde, V.; Lenguerrand, E.; Gooberman-Hill, R.; Beswick, A.D.; Marques, E.; Noble, S.; Horwood, J.; Pyke, M.; Dieppe, P.; Blom, A.W. Effect of Local Anaesthetic Infiltration on Chronic Postsurgical Pain after Total Hip and Knee Replacement: The APEX Randomised Controlled Trials. Pain 2015, 156, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, E.J.; Levene, J.L.; Cohen, M.S.; Andreae, D.A.; Chao, J.Y.; Johnson, M.; Hall, C.B.; Andreae, M.H. Local Anaesthetics and Regional Anaesthesia versus Conventional Analgesia for Preventing Persistent Postoperative Pain in Adults and Children. Cochrane Database Syst. Rev. 2018, 2018, CD007105. [Google Scholar] [CrossRef] [PubMed]
- Beswick, A.D.; Dennis, J.; Gooberman-Hill, R.; Blom, A.W.; Wylde, V. Are Perioperative Interventions Effective in Preventing Chronic Pain after Primary Total Knee Replacement? A Systematic Review. BMJ Open 2019, 9, e028093. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Ren, L.; Qin, P.; Chen, J.; Feng, P.; Lin, H.; Su, M. Continuous Femoral Nerve Block versus Intravenous Patient Controlled Analgesia for Knee Mobility and Long-Term Pain in Patients Receiving Total Knee Replacement: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. ECAM 2014, 2014, 569107. [Google Scholar] [CrossRef] [PubMed]
- Jogie, J.; Jogie, J.A. A Comprehensive Review on the Efficacy of Nerve Blocks in Reducing Postoperative Anesthetic and Analgesic Requirements. Cureus 2023, 15, e38552. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chelly, J.E.; Williams, J.; Gold, M.S. Impact of Peripheral Nerve Block with Low Dose Local Anesthetics on Analgesia and Functional Outcomes Following Total Knee Arthroplasty: A Retrospective Study. Pain Med. Malden Mass 2015, 16, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Sarpong, N.O.; Lakra, A.; Jennings, E.; Cooper, H.J.; Shah, R.P.; Geller, J.A. Same-Day Physical Therapy Following Total Knee Arthroplasty Leads to Improved Inpatient Physical Therapy Performance and Decreased Inpatient Opioid Consumption. J. Arthroplast. 2019, 34, 2931–2936. [Google Scholar] [CrossRef] [PubMed]
- Mion, G.; Villevieille, T. Ketamine Pharmacology: An Update (Pharmacodynamics and Molecular Aspects, Recent Findings). CNS Neurosci. Ther. 2013, 19, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Yavi, M.; Lee, H.; Henter, I.D.; Park, L.T.; Zarate, C.A. Ketamine Treatment for Depression: A Review. Discov. Ment. Health 2022, 2, 9. [Google Scholar] [CrossRef]
- Vadivelu, N.; Schermer, E.; Kodumudi, V.; Belani, K.; Urman, R.D.; Kaye, A.D. Role of Ketamine for Analgesia in Adults and Children. J. Anaesthesiol. Clin. Pharmacol. 2016, 32, 298–306. [Google Scholar] [CrossRef]
- Orser, B.A.; Pennefather, P.S.; MacDonald, J.F. Multiple Mechanisms of Ketamine Blockade of N-Methyl-D-Aspartate Receptors. Anesthesiology 1997, 86, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Stubhaug, A.; Breivik, H.; Eide, P.K.; Kreunen, M.; Foss, A. Mapping of Punctuate Hyperalgesia around a Surgical Incision Demonstrates That Ketamine Is a Powerful Suppressor of Central Sensitization to Pain Following Surgery. Acta Anaesthesiol. Scand. 1997, 41, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Remérand, F.; Le Tendre, C.; Baud, A.; Couvret, C.; Pourrat, X.; Favard, L.; Laffon, M.; Fusciardi, J. The Early and Delayed Analgesic Effects of Ketamine after Total Hip Arthroplasty: A Prospective, Randomized, Controlled, Double-Blind Study. Anesth. Analg. 2009, 109, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Perrin, S.B.; Purcell, A.N. Intraoperative Ketamine May Influence Persistent Pain Following Knee Arthroplasty under Combined General and Spinal Anaesthesia: A Pilot Study. Anaesth. Intensive Care 2009, 37, 248–253. [Google Scholar] [CrossRef]
- Aveline, C.; Roux, A.L.; Hetet, H.L.; Gautier, J.F.; Vautier, P.; Cognet, F.; Bonnet, F. Pain and Recovery After Total Knee Arthroplasty: A 12-Month Follow-up After a Prospective Randomized Study Evaluating Nefopam and Ketamine for Early Rehabilitation. Clin. J. Pain 2014, 30, 749. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, X.; Mu, Y.; Li, Q.; Liu, J. A Review of the Mechanism of the Central Analgesic Effect of Lidocaine. Medicine 2020, 99, e19898. [Google Scholar] [CrossRef] [PubMed]
- Doleman, B.; Mathiesen, O.; Sutton, A.J.; Cooper, N.J.; Lund, J.N.; Williams, J.P. Non-Opioid Analgesics for the Prevention of Chronic Postsurgical Pain: A Systematic Review and Network Meta-Analysis. BJA Br. J. Anaesth. 2023, 130, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Liu, C.-L.; Liu, T.-P.; Yang, P.-S.; Chen, M.-J.; Cheng, S.-P. Effect of Perioperative Intravenous Lidocaine Infusion on Acute and Chronic Pain after Breast Surgery: A Meta-Analysis of Randomized Controlled Trials. Pain Pract. Off. J. World Inst. Pain 2017, 17, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.; Corcoran, T.; Schug, S.; Toner, A. Perioperative Lidocaine Infusions for the Prevention of Chronic Postsurgical Pain: A Systematic Review and Meta-Analysis of Efficacy and Safety. Pain 2018, 159, 1696–1704. [Google Scholar] [CrossRef]
- Marks, D.M.; Shah, M.J.; Patkar, A.A.; Masand, P.S.; Park, G.-Y.; Pae, C.-U. Serotonin-Norepinephrine Reuptake Inhibitors for Pain Control: Premise and Promise. Curr. Neuropharmacol. 2009, 7, 331–336. [Google Scholar] [CrossRef]
- Schnabel, A.; Weibel, S.; Reichl, S.U.; Meißner, M.; Kranke, P.; Zahn, P.K.; Pogatzki-Zahn, E.M.; Meyer-Frießem, C.H. Efficacy and Adverse Events of Selective Serotonin Noradrenaline Reuptake Inhibitors in the Management of Postoperative Pain: A Systematic Review and Meta-Analysis. J. Clin. Anesth. 2021, 75, 110451. [Google Scholar] [CrossRef]
- Rienstra, W.; Blikman, T.; Dijkstra, B.; Stewart, R.; Zijlstra, W.; van Raaij, T.; Ten Hagen, A.; Bulstra, S.; Stevens, M.; van den Akker-Scheek, I. Effect of Preoperative Duloxetine Treatment on Postoperative Chronic Residual Pain after Total Hip or Knee Arthroplasty: A Randomised Controlled Trial. BMJ Open 2021, 11, e052944. [Google Scholar] [CrossRef] [PubMed]
- YaDeau, J.T.; Brummett, C.M.; Mayman, D.J.; Lin, Y.; Goytizolo, E.A.; Padgett, D.E.; Alexiades, M.M.; Kahn, R.L.; Jules-Elysee, K.M.; Fields, K.G.; et al. Duloxetine and Subacute Pain after Knee Arthroplasty When Added to a Multimodal Analgesic Regimen: A Randomized, Placebo-Controlled, Triple-Blinded Trial. Anesthesiology 2016, 125, 561–572. [Google Scholar] [CrossRef]
- Wang, L.; Tobe, J.; Au, E.; Tran, C.; Jomy, J.; Oparin, Y.; Couban, R.J.; Paul, J. Selective Serotonin Reuptake Inhibitors and Serotonin–Norepinephrine Reuptake Inhibitors as Adjuncts for Postoperative Pain Management: Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Anaesth. 2022, 128, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Walker, A.M.; Premji, Z.A.; Beauchemin-Turcotte, M.-E.; Wong, J.; Soh, S.; Hawboldt, G.S.; Shinkaruk, K.S.; Archer, D.P. Preventing Persistent Postsurgical Pain: A Systematic Review and Component Network Meta-Analysis. Eur. J. Pain 2022, 26, 771–785. [Google Scholar] [CrossRef]
- Moucha, C.S.; Weiser, M.C.; Levin, E.J. Current Strategies in Anesthesia and Analgesia for Total Knee Arthroplasty. JAAOS—J. Am. Acad. Orthop. Surg. 2016, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Dickenson, A.H. Mechanisms of the Gabapentinoids and α 2 Δ-1 Calcium Channel Subunit in Neuropathic Pain. Pharmacol. Res. Perspect. 2016, 4, e00205. [Google Scholar] [CrossRef]
- Buvanendran, A.; Kroin, J.S.; Della Valle, C.J.; Kari, M.; Moric, M.; Tuman, K.J. Perioperative Oral Pregabalin Reduces Chronic Pain after Total Knee Arthroplasty: A Prospective, Randomized, Controlled Trial. Anesth. Analg. 2010, 110, 199–207. [Google Scholar] [CrossRef]
- YaDeau, J.T.; Lin, Y.; Mayman, D.J.; Goytizolo, E.A.; Alexiades, M.M.; Padgett, D.E.; Kahn, R.L.; Jules-Elysee, K.M.; Ranawat, A.S.; Bhagat, D.D.; et al. Pregabalin and Pain after Total Knee Arthroplasty: A Double-Blind, Randomized, Placebo-Controlled, Multidose Trial. BJA Br. J. Anaesth. 2015, 115, 285–293. [Google Scholar] [CrossRef]
- Clarke, H.; Bonin, R.P.; Orser, B.A.; Englesakis, M.; Wijeysundera, D.N.; Katz, J. The Prevention of Chronic Postsurgical Pain Using Gabapentin and Pregabalin: A Combined Systematic Review and Meta-Analysis. Anesth. Analg. 2012, 115, 428–442. [Google Scholar] [CrossRef]
- Kim, B.R.; Yoon, S.-H.; Lee, H.-J. Practical Strategies for the Prevention and Management of Chronic Postsurgical Pain. Korean J. Pain 2023, 36, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Vergne-Salle, P. Management of Neuropathic Pain after Knee Surgery. Joint Bone Spine 2016, 83, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Früh, A.; Sargut, T.A.; Hussein, A.; Muskala, B.; Kuckuck, A.; Brüßeler, M.; Vajkoczy, P.; Bayerl, S. Peripheral Nerve Stimulation for the Treatment of Chronic Knee Pain. Sci. Rep. 2023, 13, 15543. [Google Scholar] [CrossRef] [PubMed]
- Qudsi-Sinclair, S.; Borrás-Rubio, E.; Abellan-Guillén, J.F.; Padilla del Rey, M.L.; Ruiz-Merino, G. A Comparison of Genicular Nerve Treatment Using Either Radiofrequency or Analgesic Block with Corticosteroid for Pain after a Total Knee Arthroplasty: A Double-Blind, Randomized Clinical Study. Pain Pract. 2017, 17, 578–588. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, J.; Pak, S.; Shteynman, L.; Winkeler, I.; Jin, Z.; Kaczocha, M.; Bergese, S.D. Mechanisms and Preventative Strategies for Persistent Pain following Knee and Hip Joint Replacement Surgery: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4722. https://doi.org/10.3390/ijms25094722
Murphy J, Pak S, Shteynman L, Winkeler I, Jin Z, Kaczocha M, Bergese SD. Mechanisms and Preventative Strategies for Persistent Pain following Knee and Hip Joint Replacement Surgery: A Narrative Review. International Journal of Molecular Sciences. 2024; 25(9):4722. https://doi.org/10.3390/ijms25094722
Chicago/Turabian StyleMurphy, Jasper, Sery Pak, Lana Shteynman, Ian Winkeler, Zhaosheng Jin, Martin Kaczocha, and Sergio D. Bergese. 2024. "Mechanisms and Preventative Strategies for Persistent Pain following Knee and Hip Joint Replacement Surgery: A Narrative Review" International Journal of Molecular Sciences 25, no. 9: 4722. https://doi.org/10.3390/ijms25094722
APA StyleMurphy, J., Pak, S., Shteynman, L., Winkeler, I., Jin, Z., Kaczocha, M., & Bergese, S. D. (2024). Mechanisms and Preventative Strategies for Persistent Pain following Knee and Hip Joint Replacement Surgery: A Narrative Review. International Journal of Molecular Sciences, 25(9), 4722. https://doi.org/10.3390/ijms25094722