Building Triazolated Macrocycles from Bis-Propargylated Calix[4]arenes and Bis-Azidomethylated Azobenzene or Stilbene
Abstract
:1. Introduction
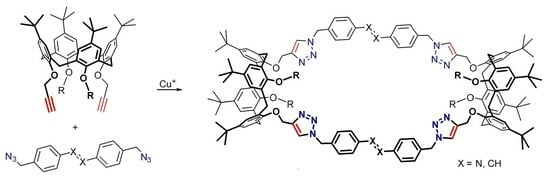
2. Results and Discussion
3. Materials and Methods
3.1. (E)-1,2-bis(4-(azidomethyl)phenyl)ethene 4
3.2. Bis(calixarene) 5 and Cyclic Oligomer c5
3.3. Bis(calixarene) 6 and Cyclic Oligomer c6
3.4. Bis(calixarene) 8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escobar, L.; Sun, Q.; Ballester, P. Aryl-Extended and Super Aryl-Extended Calix[4]pyrroles: Design, Synthesis, and Applications. Acc. Chem. Res. 2023, 56, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Wagay, S.A.; Khan, L.; Ali, R. Recent Advancements in Ion-Pair Receptors. Chem. Asian J. 2023, 18, e202201080. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-L.; Yu, S.-Q.; Huang, X.-H.; Gong, H.-Y. Bismacrocycle: Structures and Applications. Molecules 2023, 28, 6043. [Google Scholar] [CrossRef] [PubMed]
- Hirao, T. Macromolecular architectures constructed by biscalix[5]arene–[60]fullerene host–guest interactions. Polym. J. 2023, 55, 95–104. [Google Scholar] [CrossRef]
- Rather, I.A.; Ali, R.; Ali, A. Recent developments in calix[4]pyrrole (C4P)-based supramolecular functional systems. Org. Chem. Front. 2022, 9, 6416–6440. [Google Scholar] [CrossRef]
- Liu, Z.; Li, B.; Song, L.; Zhang, H. Pillar[n]arene–calix[m]arene hybrid macrocyclic structures. RSC Adv. 2022, 12, 28185–28195. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Han, J. Crown ether–pillararene hybrid macrocyclic systems. Org. Biomol. Chem. 2021, 19, 3287–3302. [Google Scholar] [CrossRef]
- Shimoyama, D.; Haino, T. Feet-to-Feet-Connected Multitopic Resorcinarene Macrocycles. Asian J. Org. Chem. 2020, 9, 1718–1725. [Google Scholar] [CrossRef]
- Peng, S.; He, Q.; Vargas-Zúñiga, G.I.; Qin, L.; Hwang, I.; Kim, S.K.; Heo, N.J.; Lee, C.-H.; Dutta, R.; Sessler, J.L. Strapped calix[4]pyrroles: From syntheses to applications. Chem. Soc. Rev. 2020, 49, 865–907. [Google Scholar] [CrossRef]
- Lai, Z.; Zhao, T.; Sessler, J.L.; He, Q. Bis–Calix[4]pyrroles: Preparation, structure, complexation properties and beyond. Coord. Chem. Rev. 2020, 425, 213528. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Fu, H. Pillararenes Trimer for Self-Assembly. Nanomaterials 2020, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Vargas-Zúñiga, G.I.; Kim, S.H.; Kim, S.K.; Sessler, J.L. Macrocycles as Ion Pair Receptors. Chem. Rev. 2019, 119, 9753–9835. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Moscoso, A.; Ballester, P. Light-responsive molecular containers. Chem. Commun. 2017, 53, 4635–4652. [Google Scholar] [CrossRef]
- Lavendomme, R.; Moerkerke, S.; Mariaule, G.; Jabin, I. Selective binding of oxalate by a tris-ureido calix[6]tube in a protic environment. Org. Biomol. Chem. 2023, 21, 6730–6737. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.; Rodolico, R.; Fragalà, M.E.; Pappalardo, A.; Pisagatti, I.; Gattuso, G.; Notti, A.; Parisi, M.F.; Purrello, R.; D’Urso, A. Self-Assembly of Discrete Porphyrin/Calix[4]tube Complexes Promoted by Potassium Ion Encapsulation. Molecules 2021, 26, 704. [Google Scholar] [CrossRef] [PubMed]
- Vita, F.; Vorti, M.; Orlandini, G.; Zanichelli, V.; Massera, C.; Ugozzoli, F.; Arduini, A.; Secchi, A. Synthesis and recognition properties of calix[4]arene semitubes as ditopic hosts for N-alkylpyridinium ion pairs. CrystEngComm 2016, 18, 5017–5027. [Google Scholar] [CrossRef]
- Puchnin, K.; Zaikin, P.; Cheshkov, D.; Vatsouro, I.; Kovalev, V. Calix[4]tubes: An Approach to Functionalization. Chem. Eur. J. 2012, 18, 10954–10968. [Google Scholar] [CrossRef]
- Begel, S.; Puchta, R.; van Eldik, R. Host-guest complexes of calix[4]tubes-prediction of ion selectivity by quantum chemical calculations VI. J. Mol. Model. 2014, 20, 2200. [Google Scholar] [CrossRef]
- Mizyed, S.; Marji, D.; Rawashdeh, A.M.; Foudeh, A. Synthesis of p-tert-butylcalix[4]arene semitubes and their binding studies with C60. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 113–118. [Google Scholar] [CrossRef]
- Yang, F.F.; Liu, L.M.; Liu, C.H.; Zheng, X.H.; Guo, Y. Synthesis of novel biscalixarene-tube containing two kinds of calix[4]arene derivative units. Chin. Chem. Lett. 2008, 19, 9–11. [Google Scholar] [CrossRef]
- Morales-Sanfrutos, J.; Ortega-Muñoz, M.; Lopez-Jaramillo, J.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F. Synthesis of Calixarene-Based Cavitands and Nanotubes by Click Chemistry. J. Org. Chem. 2008, 73, 7768–7771. [Google Scholar] [CrossRef]
- Organo, V.G.; Sgarlata, V.; Firouzbakht, F.; Rudkevich, D.M. Long Synthetic Nanotubes from Calix[4]arenes. Chem. Eur. J. 2007, 13, 4014–4023. [Google Scholar] [CrossRef] [PubMed]
- Organo, V.G.; Rudkevich, D.M. Emerging host–guest chemistry of synthetic nanotubes. Chem. Commun. 2007, 3891–3899. [Google Scholar] [CrossRef] [PubMed]
- Organo, V.G.; Leontiev, A.V.; Sgarlata, V.; Dias, H.V.R.; Rudkevich, D.M. Supramolecular Features of Calixarene-Based Synthetic Nanotubes. Angew. Chem. Int. Ed. 2005, 44, 3043–3047. [Google Scholar] [CrossRef] [PubMed]
- Sgarlata, V.; Organo, V.G.; Rudkevich, D.M. A procedure for filling calixarene nanotubes. Chem. Commun. 2005, 5630–5632. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, S.H.; Kwon, S.-G.; Lee, E.-H.; Lee, S.H.; Kim, J.S. Synthesis of a New Calix[4]crown-5-azacrown-5 Dimeric Nano-tube. Bull. Korean Chem. Soc. 2004, 25, 1244–1246. [Google Scholar] [CrossRef]
- Zyryanov, G.V.; Rudkevich, D.M. Toward Synthetic Tubes for NO2/N2O4: Design, Synthesis, and Host−Guest Chemistry. J. Am. Chem. Soc. 2004, 126, 4264–4270. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Lee, J.K.; Lee, S.H.; Lim, M.S.; Lee, S.W.; Sim, W.; Kim, J.S. Silver Ion Shuttling in the Trimer-Mimic Thiacalix[4]crown Tube. J. Org. Chem. 2004, 69, 2877–2880. [Google Scholar] [CrossRef]
- Webber, P.R.A.; Cowley, A.; Drew, M.G.B.; Beer, P.D. Potassium Selective Calix[4]semitubes. Chem. Eur. J. 2003, 9, 2439–2446. [Google Scholar] [CrossRef]
- Webber, P.R.A.; Beer, P.D. Ion-pair recognition by a ditopic calix[4]semitube receptor. Dalton Trans. 2003, 2249–2252. [Google Scholar] [CrossRef]
- Matthews, S.E.; Schmitt, P.; Felix, V.; Drew, M.G.B.; Beer, P.D. Calix[4]tubes: A New Class of Potassium-Selective Ionophore. J. Am. Chem. Soc. 2002, 124, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Lentin, I.; Gorbunov, A.; Bezzubov, S.; Nosova, V.; Cheshkov, D.; Kovalev, V.; Vatsouro, I. Shrinkable/stretchable bis(calix[4]arenes) comprising photoreactive azobenzene or stilbene linkers. Org. Chem. Front. 2023, 10, 1470–1484. [Google Scholar] [CrossRef]
- Gorbunov, A.; Cheshkov, D.; Kovalev, V.; Vatsouro, I. Copper(I)-Catalyzed Cycloaddition of Azides to Multiple Alkynes: A Selectivity Study Using a Calixarene Framework. Chem. Eur. J. 2015, 21, 9528–9534. [Google Scholar] [CrossRef] [PubMed]
- Gorbunov, A.; Kuznetsova, J.; Deltsov, I.; Molokanova, A.; Cheshkov, D.; Bezzubov, S.; Kovalev, V.; Vatsouro, I. Selective azide–alkyne cycloaddition reactions of azidoalkylated calixarenes. Org. Chem. Front. 2020, 7, 2432–2441. [Google Scholar] [CrossRef]
- Gorbunov, A.; Kuznetsova, J.; Puchnin, K.; Kovalev, V.; Vatsouro, I. Triazolated calix[4]arenes from 2-azidoethylated precursors: Is there a difference in the way the triazoles are attached to narrow rims? New J. Chem. 2019, 43, 4562–4580. [Google Scholar] [CrossRef]
- Bezzubov, S.; Ermolov, K.; Gorbunov, A.; Kalle, P.; Lentin, I.; Latyshev, G.; Kovalev, V.; Vatsouro, I. Inherently dinuclear iridium(III) meso architectures accessed by cyclometalation of calix[4]arene-based bis(aryltriazoles). Dalton Trans. 2021, 50, 16765–16769. [Google Scholar] [CrossRef]
- Gorbunov, A.; Ozerov, N.; Malakhova, M.; Eshtukov, A.; Cheshkov, D.; Bezzubov, S.; Kovalev, V.; Vatsouro, I. Assembling triazolated calix[4]semitubes by means of copper(I)-catalyzed azide–alkyne cycloaddition. Org. Chem. Front. 2021, 8, 3853–3866. [Google Scholar] [CrossRef]
- Malakhova, M.; Gorbunov, A.; Lentin, I.; Kovalev, V.; Vatsouro, I. Switchable silver-ion complexation by triazolated calix[4]semitubes. Org. Biomol. Chem. 2022, 20, 8092–8103. [Google Scholar] [CrossRef]
- Cecioni, S.; Lalor, R.; Blanchard, B.; Praly, J.-P.; Imberty, A.; Matthews, S.E.; Vidal, S. Achieving High Affinity towards a Bacterial Lectin through Multivalent Topological Isomers of Calix[4]arene Glycoconjugates. Chem. Eur. J. 2009, 15, 13232–13240. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yoshikoshi, K.; Aoki, T.; Yamagishi, T.-A. Photoreversible switching between assembly and disassembly of a supramolecular polymer involving an azobenzene-bridged pillar[5]arene dimer. Chem. Commun. 2013, 49, 8785–8787. [Google Scholar] [CrossRef]
- Freydank, A.C.; Humphrey, M.G.; Friedrich, R.W.; Luther-Davies, B. Synthesis and optical characterization of unsymmetrical oligophenylenevinylenes. Tetrahedron 2002, 58, 1425–1432. [Google Scholar] [CrossRef]
- Sakovich, M.; Sokolova, D.; Alekseev, I.; Lentin, I.; Gorbunov, A.; Malakhova, M.; Ershov, I.; Zairov, R.; Korniltsev, I.; Podyachev, S.; et al. Enriching calixarene functionality with 1,3-diketone groups. Org. Chem. Front. 2023, 10, 3619–3636. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. 2015, C71, 9–18. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lentin, I.; Korniltsev, I.; Gorbunov, A.; Cheshkov, D.; Bezzubov, S.; Kovalev, V.; Vatsouro, I. Building Triazolated Macrocycles from Bis-Propargylated Calix[4]arenes and Bis-Azidomethylated Azobenzene or Stilbene. Molbank 2023, 2023, M1748. https://doi.org/10.3390/M1748
Lentin I, Korniltsev I, Gorbunov A, Cheshkov D, Bezzubov S, Kovalev V, Vatsouro I. Building Triazolated Macrocycles from Bis-Propargylated Calix[4]arenes and Bis-Azidomethylated Azobenzene or Stilbene. Molbank. 2023; 2023(4):M1748. https://doi.org/10.3390/M1748
Chicago/Turabian StyleLentin, Ivan, Ilia Korniltsev, Alexander Gorbunov, Dmitry Cheshkov, Stanislav Bezzubov, Vladimir Kovalev, and Ivan Vatsouro. 2023. "Building Triazolated Macrocycles from Bis-Propargylated Calix[4]arenes and Bis-Azidomethylated Azobenzene or Stilbene" Molbank 2023, no. 4: M1748. https://doi.org/10.3390/M1748
APA StyleLentin, I., Korniltsev, I., Gorbunov, A., Cheshkov, D., Bezzubov, S., Kovalev, V., & Vatsouro, I. (2023). Building Triazolated Macrocycles from Bis-Propargylated Calix[4]arenes and Bis-Azidomethylated Azobenzene or Stilbene. Molbank, 2023(4), M1748. https://doi.org/10.3390/M1748