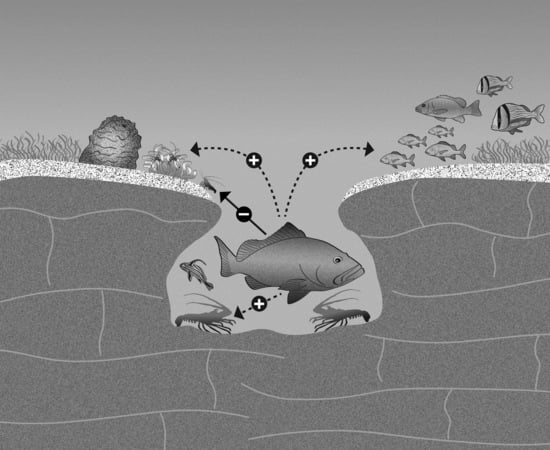
Red Grouper (Epinephelus morio) Shape Faunal Communities via Multiple Ecological Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Statistical Analysis
3. Results
4. Discussion
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Family | Species | Common Name | Functional Group | |
|---|---|---|---|---|
| Feeding | Habitat | |||
| Acanthuridae | Acanthurus chirurgus | Doctorfish | BA | MILL |
| Acanthurus coeruleus | Blue Tang | BA | MILL | |
| Apogonidae | Apogon binotatus | Barred Cardinalfish | PL | DEM |
| Apogon maculatus | Flamefish | ZB | DEM | |
| Balistidae | Balistes capriscus | Gray Triggerfish | ZB | MILL |
| Chaetodontidae | Chaetodon ocellatus | Spotfin Butterflyfish | ZB | MILL |
| Chaetodon sedentarius | Reef Butterflyfish | ZB | MILL | |
| Gerreidae | Eucinostomus melanopterus | Flagfin Mojarra | ZB | TRANS |
| Ginglymostomatidae | Ginglymostoma cirratum | Nurse Shark | INV | DEM |
| Gobiidae | Coryphopterus glaucofraenum | Bridled Goby | BA | DEM |
| Elacatinus oceanops | Neon Goby | CL | DEM | |
| Haemulidae | Anisotremus virginicus | Porkfish | CL/ZB | MILL |
| Haemulon chrysargyreum | Smallmouth Grunt | ZB | MILL | |
| Haemulon flavolineatum | French Grunt | ZB | MILL | |
| Haemulon plumierii | White Grunt | ZB | MILL | |
| Haemulon sciurus | Bluestriped Grunt | ZB | MILL | |
| Labridae | Lachnolaimus maximus | Hogfish | ZB | TRANS |
| Thalassoma bifasciatum | Bluehead Wrasse | CL/PL | TRANS | |
| Lutjanidae | Lutjanus griseus | Gray Snapper | ZB | TRANS |
| Lutjanus synagris | Lane Snapper | ZB | TRANS | |
| Ocyurus chrysurus | Yellowtail Snapper | PV | TRANS | |
| Mullidae | Pseudupeneus maculatus | Spotted Goatfish | ZB | TRANS |
| Muraenidae | Gymnothorax funebris | Green Moray | INV/PV | DEM |
| Gymnothorax vicinus | Purplemouth Moray | INV/PV | DEM | |
| Pomacanthidae | Holacanthus bermudensis | Blue Angelfish | ZB | MILL |
| Holacanthus ciliaris | Queen Angelfish | ZB | MILL | |
| Pomacanthus arcuatus | Gray Angelfish | ZB | MILL | |
| Pomacanthus paru | French Angelfish | CL/ZB | MILL | |
| Pomacentridae | Abudefdef saxatilis | Sergeant Major | ZB | MILL |
| Scaridae | Scarus coeruleus | Blue Parrotfish | BA | TRANS |
| Scarus iseri | Striped Parrotfish | BA | TRANS | |
| Sparisoma aurofrenatum | Redband Parrotfish | BA | TRANS | |
| Sciaenidae | Equetus lanceolatus | Jacknife Fish | ZB | DEM |
| Pareques acuminatus | High-Hat | ZB | DEM | |
| Scorpaenidae | Pterois miles/volitans | Lionfish | PV | DEM |
| Scorpaena plumieri | Spotted Scorpionfish | PV | DEM | |
| Serranidae | Diplectrum formosum | Sand Perch | ZB | TRANS |
| Epinephelus itajara | Goliath Grouper | INV | DEM | |
| Epinephelus morio | Red Grouper | INV | DEM | |
| Hypoplectrus puella | Barred Hamlet | ZB | MILL | |
| Mycteroperca bonaci | Black Grouper | PV | MILL | |
| Sparidae | Calamus calamus | Saucereye Porgy | ZB | TRANS |
| Phylum | Family | Species | Common Name | Functional Group | |
|---|---|---|---|---|---|
| RG Diet | CL | ||||
| Arthropoda | Hippolytidae | Lysmata spp. | Peppermint shrimp | N | Y |
| Majidae | Mithrax spinosissimus | Channel clinging crab | Y | N | |
| Palaemonidae | Ancylomenes pedersoni | Pederson’s cleaner shrimp | N | Y | |
| Periclimines yucatanicus | Spotted cleaner shrimp | N | Y | ||
| Palinuridae | Panulirus argus | Caribbean spiny lobster | Y | N | |
| Scyllaridae | Scyllarides aequinoctialis | Spanish lobster | Y | N | |
| Stenopodidae | Stenorhynchus seticornis | Yellowline arrow crab | N | N | |
| Xanthidae | Menippe mercenaria | Florida stone crab | N | N | |
| Echinodermata | Clypeasteridae | Clypeaster rosaceus | Inflated sea biscuit | N | N |
References
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Bruno, J.F.; Bertness, M.D. Habitat modification and facilitation in benthic marine communities. In Marine Community Ecology; Bertness, M.D., Gaines, S.D., Hay, M.E., Eds.; Sinauer Associates: Sunderland, MA, USA, 2001; pp. 201–218. [Google Scholar]
- Dayton, P.K. Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound Antarctica. In Proceedings of the Colloquium on Conservation Problems in Antarctica; Parker, B.C., Ed.; Allen Press: Lawrence, KA, USA, 1972; pp. 81–96. [Google Scholar]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Naeem, S.; Thompson, L.J.; Lawler, S.P.; Lawton, J.H.; Woodfin, R.M. Declining biodiversity can alter the performance of ecosystems. Nature 1994, 368, 734–737. [Google Scholar] [CrossRef]
- Coleman, F.C.; Williams, S.L. Overexploiting marine ecosystem engineers: Potential consequences for biodiversity. Trends Ecol. Evol. 2002, 17, 40–44. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Bruno, J.F.; Duffy, J.E. Understanding the effects of marine biodiversity on communities and ecosystems. Ann. Rev. 2007, 38, 739–766. [Google Scholar] [CrossRef]
- Jackson, D.R.; Milstrey, E.G. The Fauna of Gopher Tortoise Burrows; Florida Game and Fresh Water Fish Commission Nongame Wildlife Program Technical Report No. 5; Florida Game and Fresh Water Fish Commission: Tallahassee, FL, USA, 1989. [Google Scholar]
- Kinlaw, A.; Grasmueck, M. Evidence for and geomorphologic consequences of a reptilian ecosystem engineer: The burrowing cascade initiated by the Gopher Tortoise. Geomorphology 2011, 157–158, 108–121. [Google Scholar] [CrossRef]
- Able, K.W.; Grimes, C.B.; Jones, R.S.; Twichell, D.C. Temporal and spatial variation in habitat characteristics of tilefish (Lopholatilus chamaeloeonticefs) off the east coast of Florida. Bull. Mar. Sci. 1993, 53, 1013–1026. [Google Scholar]
- Hixon, M.A. Predation as a process structuring coral-reef fish communities. In The Ecology of Fishes on Coral Reefs; Sale, P.F., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 475–508. [Google Scholar]
- Almany, G.R.; Webster, M.S. The predation gauntlet: Early post-settlement mortality in reef fishes. Coral Reefs 2006, 25, 19–22. [Google Scholar] [CrossRef]
- Stallings, C.D. Predator identity and recruitment of coral-reef fishes: Indirect effects of fishing. Mar. Ecol. Prog. Ser. 2009, 383, 251–259. [Google Scholar] [CrossRef]
- Stier, A.C.; Geange, S.W.; Hanson, K.M.; Bolker, B.M. Predator density and timing of arrival affect reef fish community assembly. Ecology 2013, 94, 1057–1068. [Google Scholar] [CrossRef]
- Heinlein, J.M.; Stier, A.C.; Steele, M.A. Predators reduce abundance and species richness of coral reef fish recruits via non-selective predation. Coral Reefs 2010, 29, 527–532. [Google Scholar] [CrossRef]
- Albins, M.A. Effects of invasive Pacific red lionfish Pterois volitans versus a native predator on Bahamian coral-reef fish communities. Biol. Invasions 2013, 15, 29–43. [Google Scholar] [CrossRef]
- Keough, M.J.; Downes, B.J. Recruitment of marine invertebrates: The role of active larval choice and early mortality. Oecologia 1982, 54, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Beukers, J.S.; Jones, G.P. Habitat complexity modifies the impact of piscivores on a coral reef fish population. Oceologia 1997, 114, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 2001, 26, 32–46. [Google Scholar]
- Hixon, M.A.; Carr, M.H. Synergistic predation, density dependence, and population regulation in marine fish. Science 1997, 277, 946–949. [Google Scholar] [CrossRef]
- Stallings, C.D. Indirect effects of an exploited predator on recruitment of coral-reef fishes. Ecology 2008, 89, 2090–2095. [Google Scholar] [CrossRef]
- Paine, R.T. Food web complexity and species diversity. Am. Nat. 1966, 100, 65–75. [Google Scholar] [CrossRef]
- Paine, R.T. A note on trophic complexity and community stability. Am. Nat. 1969, 103, 91–93. [Google Scholar] [CrossRef]
- McLean, J.H. Sublittoral ecology of kelp beds of the open coast near Carmel, California. Biol. Bull. 1962, 122, 95–114. [Google Scholar] [CrossRef]
- Estes, J.A.; Palmisano, J.F. Sea otters: Their role in structuring nearshore communities. Science 1974, 185, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.A.; Smith, N.S.; Palmisano, J.F. Sea otter predation and community organization in the Western Aleutian Islands, Alaska. Ecology 1978, 59, 822–833. [Google Scholar] [CrossRef]
- Estes, J.A.; Duggins, D.O. Sea otters and kelp forests in Alaska: Generality and variation in a community ecological paradigm. Ecol. Monogr. 1995, 65, 75–100. [Google Scholar] [CrossRef]
- Coleman, F.C.; Koenig, C.C.; Scanlon, K.M.; Heppell, S.; Heppell, S.; Miller, M.W. Benthic habitat modification through excavation by red grouper, Epinephelus morio, in the northeastern Gulf of Mexico. Open Fish Sci. J. 2010, 3, 1–15. [Google Scholar] [CrossRef]
- Ellis, R.D.; Coleman, F.C.; Koenig, C.C. Effects of habitat manipulation by red grouper, Epinephelus morio, on faunal communities associated with excavations in Florida Bay. Bull. Mar. Sci. 2017, 93, 961–983. [Google Scholar] [CrossRef]
- Herrnkind, W.F.; Butler IV, M.J.; Hunt, J.H.; Childress, M. Role of physical refugia: Implications from a mass sponge die-off in a lobster nursery in Florida. Mar. Fresh. Res. 1997, 48, 759–769. [Google Scholar] [CrossRef]
- Randall, J.E. Food habits of reef fishes of the West Indies. Stud. Trop. Ocean. 1967, 5, 665–847. [Google Scholar]
- Moe, M.A. Biology of the Red Grouper (Epinephelus morio Valenciennes) from the Eastern Gulf of Mexico; Professional Papers Series; Florida Department of Natural Resources, Marine Science Laboratory: St. Petersburg, FL, USA, 1967. [Google Scholar]
- Bullock, L.H.; Smith, G.B. Seabasses (Pisces: Serranidae). Memoirs of the Hourglass Cruises,X; Florida Marine Research Institute, Department of Natural Resources: St. Petersburg, FL, USA, 1991; Volume VIII, Part II. [Google Scholar]
- Brulé, T.; Rodriguez Canche, L.G. Food habits of juvenile red groupers, Epinephelus morio (Valenciennes, 1828), from Campeche Bank, Yucatan, Mexico. Bull. Mar. Sci. 1993, 52, 772–779. [Google Scholar]
- Weaver, D.C. Feeding Ecology and Ecomorphology of Three Sea Basses (Pisces: Serranidae) in the Gulf of Mexico. Master’s thesis, University of Florida, Gainesville, FL, USA, 1996. [Google Scholar]
- Brulé, T.; Avila, D.O.; Crespo, M.S.; Deniel, C. Seasonal and diel changes in diet composition of juvenile red grouper (Epinephelus morio) from Campeche Bank. Bull. Mar. Sci. 1994, 55, 255–262. [Google Scholar]
- Hacker, S.; Gaines, S.D. Some implications of direct positive interactions for community species diversity. Ecology 1997, 78, 1990–2003. [Google Scholar] [CrossRef]
- Dill, L.M.; Heithaus, M.R.; Walters, C.J. Behaviorally mediated indirect interactions in marine communities and their conservation implications. Ecology 2003, 84, 1151–1157. [Google Scholar] [CrossRef]
- Ellis, R.D. Ecological effects of Red Grouper (Epinephelus morio) in Florida Bay. Ph.D. Thesis, Florida State University, Tallahassee, FL, USA, 2015. [Google Scholar]
- Ellis, R.D.; Faletti, M.E. Native grouper indirectly ameliorates the negative effects of invasive lionfish. Mar. Ecol. Prog. Ser. 2016, 558, 267–279. [Google Scholar] [CrossRef] [Green Version]
- Rhyne, A.L.; Lin, J. A western Atlantic peppermint shrimp complex: Redescription of Lysmata wurdemanni, description of four new species, and remarks on Lysmata rathbunae (Crustacea: Decapoda: Hipploytidae). Bull. Mar. Sci. 2006, 79, 165–204. [Google Scholar]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Hill, M.O. An evenness statistic based on the abundance-weighted variance of species proportions. Oikos 1997, 79, 413–416. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities, 1st ed.; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H. Vegan: Community Ecology Package. R package version 2.0-10, 2013. Available online: https://CRAN.R-project.org/package=vegan (accessed on 13 September 2013).
- Paine, R.T. Food-web analysis through field measurement of per capita interaction strength. Nature 1992, 355, 73–75. [Google Scholar] [CrossRef]
- Berlow, E.L.; Navarrete, S.A.; Briggs, C.J.; Power, M.E.; Menge, B.A. Quantifying variation in the strengths of species interactions. Ecology 1999, 80, 2206–2224. [Google Scholar] [CrossRef]
- Hesterberg, T. Resample: Resampling Functions. R package version 0.4, 2015. Available online: https://CRAN.R-project.org/package=resample (accessed on 25 April 2015).
- Hedges, L.V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Behav. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Nagkawa, S.; Cuthill, I.C. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef]
- Osenberg, C.W.; Sarnelle, O.; Cooper, S.D. Effect size in ecological experiments: The application of biological models in meta-analysis. Am. Nat. 1997, 150, 798–812. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. Available online: http://www.fishbase.org (accessed on 25 April 2015).
- Kirby, K.N.; Gerlanc, D. BootES: An R package for bootstrap confidence intervals on effect sizes. Behav. Res. Meth. 2013, 45, 905–927. [Google Scholar] [CrossRef] [PubMed]
- Ellison, A.M. Partitioning diversity. Ecology 2010, 91, 1962–1963. [Google Scholar] [CrossRef] [PubMed]
- Jost, L. Independence of alpha and beta diversities. Ecology 2010, 91, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, R.D.; Cox, C. Sanctuary roles in population and reproductive dynamics of Caribbean spiny lobster. In Spatial Processes and Management of Marine Populations; Kruse, G.H., Bez, N., Booth, A., Dorn, M.W., Hills, S., Lipcius, R.N., Pelletier, D., Roy, C., Smith, S.J., Witherells, D., Eds.; University of Alaska Sea Grant: Fairbanks, AK, USA, 2001; pp. 591–605. [Google Scholar]
- Ellis, R.D. Species interactions through ontogeny: Effects of size-selective predation by red grouper on Caribbean spiny lobster in solution holes of Florida Bay. J. Exp. Mar. Biol. Ecol. 2018, 506, 115–123. [Google Scholar] [CrossRef]
- Jones, C.G.; Gutiérrez, J.L.; Byers, J.E.; Crooks, J.A.; Lambrinos, J.G.; Talley, T.S. A framework for understanding physical ecosystem engineering by organisms. Oikos 2010, 119, 1862–1869. [Google Scholar] [CrossRef]
- Thayer, G.W.; Powell, A.B.; Hoss, D.E. Composition of larval, juvenile, and small adult fishes relative to changes in environmental conditions in Florida Bay. Estuaries 1999, 22, 518–533. [Google Scholar] [CrossRef]
- Sponaugle, S.; Paris, C.; Walter, K.D.; Kourafalou, V.; Alessandro, E.D. Observed and modeled larval settlement of a reef fish to the Florida Keys. Mar. Ecol. Prog. Ser. 2012, 453, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Silbiger, N.J.; Childress, M.J. Interspecific variation in anemone shrimp distribution and host selection in the Florida Keys (USA): Implications for marine conservation. Bull. Mar. Sci. 2008, 83, 329–345. [Google Scholar]
- Wicksten, M.K. Associations of fishes and their cleaners on coral reefs of Bonaire, Netherlands Antilles. Copeia 1995, 2, 477–481. [Google Scholar] [CrossRef]
- Zieman, J.; Fourqurean, J.W.; Iverson, R.L. Distribution, abundance and productivity of seagrasses and macroalgae in Florida Bay. Bull. Mar. Sci. 1989, 44, 292–311. [Google Scholar]







| Common Name | Species | Individual Contribution | Cumulative Contribution | SIMPER Rank | Rank Abundance |
|---|---|---|---|---|---|
| Juvenile grunts | Haemulon spp. | 0.276 | 0.276 | 1 | 3 |
| White grunt | Haemulon plumierii | 0.216 | 0.492 | 2 | 2 |
| Caribbean spiny lobster | Panulirus argus | 0.217 | 0.709 | 3 | 1 |
| French grunt | Haemulon flavolineatum | 0.072 | 0.781 | 4 | 5 |
| Pederson’s cleaner shrimp | Ancylomenes pedersoni | 0.061 | 0.842 | 5 | 4 |
| Gray snapper | Lutjanus griseus | 0.034 | 0.876 | 6 | 6 |
| Porkfish | Anisotremus virginicus | 0.015 | 0.891 | 7 | 7 |
| Channel clinging crab | Mithrax spinosissimus | 0.013 | 0.904 | 8 | 8 |
| High-hat | Pareques acuminatus | 0.012 | 0.916 | 9 | 9 |
| French angelfish | Pomacanthus arcuatus | 0.010 | 0.926 | 10 | 13 |
| Blue angelfish | Holacanthus bermudensis | 0.009 | 0.935 | 11 | 10 |
| Florida stone crab | Menippe mercenaria | 0.009 | 0.944 | 12 | 11 |
| Peppermint shrimp | Lysmata spp. | 0.007 | 0.951 | 13 | 16 |
| Queen angelfish | Holacanthus ciliaris | 0.007 | 0.958 | 14 | 15 |
| Hogfish | Lachnolaimus maximus | 0.006 | 0.964 | 15 | 14 |
| Spotted cleaner shrimp | Periclimenes yucatanicus | 0.005 | 0.969 | 16 | 19 |
| Doctorfish | Acanthurus chirurgus | 0.005 | 0.974 | 17 | 20 |
| Striped parrotfish | Scarus iseri | 0.004 | 0.978 | 18 | 17 |
| Sand perch | Diplectrum formosum | 0.004 | 0.982 | 19 | 22 |
| Bluestriped grunt | Haemulon sciurus | 0.003 | 0.985 | 20 | 18 |
| Species | Common Name | Experimental Effect [PI] | Bootstrapped Effect (± SE) | 95% CI |
|---|---|---|---|---|
| Haemulon plumierii | White grunt | 4.96 | 4.97 (± 2.38) | 0.206, 9.737 |
| Panulirus argus | Caribbean spiny lobster | 3.39 | 3.29 (± 2.57) | −1.85, 8.44 |
| Haemulon spp. | Juvenile grunts | 1.57 | 1.56 (± 1.19) | −0.811, 3.95 |
| Ancylomenes pedersoni | Pederson’s cleaner shrimp | 0.520 | 0.512 (± 0.257) | −0.0031, 1.03 |
| Lutjanus griseus | Gray snapper | 0.482 | 0.482 (± 0.255) | −0.0282, 0.992 |
| Anisotremus virginicus | Porkfish | 0.362 | 0.356 (± 0.161) | 0.0332, 0.679 |
| Haemulon flavolineatum | French grunt | 0.281 | 0.290 (± 0.449) | −0.607, 1.19 |
| Pareques acuminatus | High-hat | 0.0191 | 0.0198 (± 0.082) | −0.159, 0.198 |
| Holacanthus bermudensis | Blue angelfish | −0.0125 | −0.0119 (± 0.0408) | −0.0945, 0.0697 |
| Lachnolaimus maximus | Hogfish | −0.0333 | −0.0331 (± 0.0149) | −0.0629, −0.00329 |
| Haemulon chrysargyreum | Smallmouth grunt | −0.0375 | −0.0368 (± 0.0362) | −0.109, 0.0356 |
| Mycteroperca bonaci | Black grouper | −0.0500 | −0.0515 (± 0.0485) | −0.148, 0.0455 |
| Lutjanus synagris | Lane snapper | −0.0583 | −0.0572 (± 0.0563) | −0.170, 0.0554 |
| Scarus coeruleus | Blue parrotfish | −0.0625 | −0.0624 (± 0.0607) | −0.184, 0.0590 |
| Pomacanthus paru | French angelfish | −0.0625 | −0.0634 (± 0.0563) | −0.183, 0.0558 |
| Balistes capriscus | Gray triggerfish | −0.0625 | −0.0643 (± 0.0606) | −0.185, 0.0568 |
| Haemulon sciurus | Bluestriped grunt | −0.0667 | −0.0685 (± 0.0438) | −0.156, 0.0190 |
| Menippe mercenaria | Florida stone crab | −0.0750 | −0.0730 (± 0.0670) | −0.207, 0.0611 |
| Scarus iseri | Striped parrotfish | −0.0833 | −0.0812 (± 0.0551) | −0.191, 0.0290 |
| Holacanthus ciliaris | Queen angelfish | −0.100 | −0.0999 (± 0.0518) | −0.204, 0.00373 |
| Acanthurus chirurgus | Doctorfish | −0.104 | −0.103 (± 0.0686) | −0.241, 0.0339 |
| Equetus lanceolatus | Jackknife fish | −0.108 | −0.107 (± 0.0712) | −0.250, 0.0350 |
| Mithrax spinosissimus | Channel clinging crab | −0.145 | −0.147 (± 0.0873) | −0.321, 0.0279 |
| Ocyurus chrysurus | Yellowtail snapper | −0.163 | −0.161 (± 0.0812) | −0.323, 0.00199 |
| Pomacanthus arcuatus | Gray angelfish | −0.169 | −0.172 (± 0.0632) | −0.298, −0.0458 |
| Lysmata spp. | Peppermint shrimp | −0.175 | −0.178 (± 0.0694) | −0.317, −0.0395 |
| Diplectrum formosum | Sand perch | −0.215 | −0.211 (± 0.0911) | −0.393, −0.0290 |
| Periclimenes yucatanicus | Spotted cleaner shrimp | −0.244 | −0.244 (± 0.0794) | −0.402, −0.0848 |
| Functional Group | Effect Size g, Experimental | 95% CI | Effect Size g, Observational | 95% CI |
|---|---|---|---|---|
| All Fishes | 0.538 | −0.281, 1.09 | 1.41 | 1.11, 1.70 |
| Herbivores | 0.224 | −0.550, 1.03 | 0.626 | 0.216, 0.943 |
| Planktivores | 0.012 | −0.650, 0.823 | 1.02 | 0.792, 1.23 |
| Benthivores | 0.546 | −0.194, 1.07 | 0.645 | 0.375, 0.865 |
| Invertivores | 0.677 | 0.362, 1.11 | 0.439 | 0.043, 0.734 |
| Piscivores | 0.151 | −0.622, 0.751 | 0.276 | −0.127, 0.716 |
| Demersal Fishes | 0.469 | −0.215, 1.19 | 0.768 | 0.249, 1.23 |
| Water Column Fishes | 0.499 | −0.152, 1.10 | 1.34 | 1.07, 1.60 |
| Transient Fishes | 0.396 | −0.340, 1.04 | 0.0239 | −0.393, 0.376 |
| All Invertebrates | 0.346 | −0.446, 1.15 | 1.12 | 0.682, 1.51 |
| Red grouper Prey | 0.309 | −0.464, 1.10 | 0.771 | 0.375, 1.16 |
| Not Prey | 0.184 | −0.479, 1.05 | 0.959 | 0.561, 1.38 |
| Cleaners | 0.395 | −0.427, 1.16 | 0.993 | 0.539, 1.39 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellis, R.D. Red Grouper (Epinephelus morio) Shape Faunal Communities via Multiple Ecological Pathways. Diversity 2019, 11, 89. https://doi.org/10.3390/d11060089
Ellis RD. Red Grouper (Epinephelus morio) Shape Faunal Communities via Multiple Ecological Pathways. Diversity. 2019; 11(6):89. https://doi.org/10.3390/d11060089
Chicago/Turabian StyleEllis, Robert D. 2019. "Red Grouper (Epinephelus morio) Shape Faunal Communities via Multiple Ecological Pathways" Diversity 11, no. 6: 89. https://doi.org/10.3390/d11060089
APA StyleEllis, R. D. (2019). Red Grouper (Epinephelus morio) Shape Faunal Communities via Multiple Ecological Pathways. Diversity, 11(6), 89. https://doi.org/10.3390/d11060089