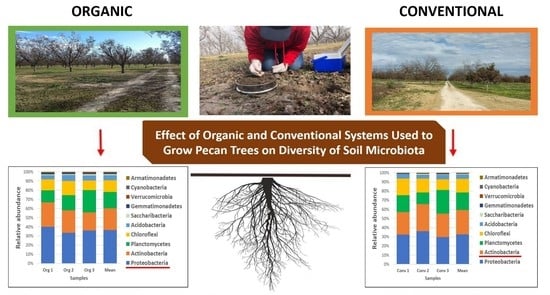
Effect of Organic and Conventional Systems Used to Grow Pecan Trees on Diversity of Soil Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling and Analysis of Chemical and Biological Variables
2.3. DNA Extraction, Amplification and Sequencing
2.4. Statistical Analysis
3. Results
3.1. Chemical and Biological Variables of the Soil
3.2. Abundance of Bacterial Taxa
4. Discussion
4.1. Chemical and Biological Variables of the Soil
4.2. Abundance of Bacterial Taxa
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Trivedi, P.; Osanai, Y.; Liu, Y.R.; Hamonts, K.; Jeffries, T.C.; Singh, B.K. Carbon Content and Climate Variability Drive Global Soil Bacterial Diversity Patterns. Ecol. Monogr. 2016, 86, 373–380. [Google Scholar] [CrossRef]
- Berthrong, S.T.; Buckley, D.H.; Drinkwater, L.E. Agricultural Management and Labile Carbon Additions Affect Soil Microbial Community Structure and Interact with Carbon and Nitrogen Cycling. Microb. Ecol. 2013, 66, 158–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, D.; Wall, D. Soil Microbiology, Ecology and Biochemistry, 4th ed.; Academic: Boston, MA, USA, 2015; pp. 11–149. [Google Scholar]
- Creamer, A.; de Menezes, B.; Krull, S.; Sanderman, J.; Newton-Walters, R.; Farrell, M. Corrigendum to “Microbial Community Structure Mediates Response of soil C Decomposition to Litter Addition and Warming. Soil Biol. Biochem. 2015, 83, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Bueno, N.G.; Valenzuela-Encinas, C.; Marsch, R.; Ortiz-Gutiérrez, D.; Verhulst, N.; Govaerts, B.; Dendooven, L.; Navarro-Noya, Y.E. Bacterial Indicator Taxa in Soils under Different Long-Term Agricultural Management. J. Appl. Microbiol. 2016, 120, 921–933. [Google Scholar] [CrossRef] [Green Version]
- Mathew, R.P.; Feng, Y.; Githinji, L.; Ankumah, R.; Balkcom, K.S. Impact of No-Tillage and Conventional Tillage Systems on Soil Microbial Communities. Appl. Environ. Soil Sci. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Van Diepeningen, A.D.; De Vos, O.J.; Korthals, G.W.; Van Bruggen, A.H.C. Effects of Organic versus Conventional Management on Chemical and Biological Parameters in Agricultural Soils. Appl. Soil Ecol. 2006, 31, 120–135. [Google Scholar] [CrossRef]
- Smith, F.P.; Prober, S.M.; House, A.P.N.; McIntyre, S. Maximizing Retention of Native Biodiversity in Australian Agricultural Landscapes-The 10:20:40:30 Guidelines. Agric. Ecosyst. Environ. 2013, 166, 35–45. [Google Scholar] [CrossRef]
- Maffei, D.F.; Batalha, E.Y.; Landgraf, M.; Schaffner, D.W.; Franco, B.D.G.M. Microbiology of Organic and Conventionally Grown Fresh Produce. Braz. J. Microbiol. 2016, 47, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Mishra, D.; Rajvir, S.; Mishra, U.; Kumar, S. Role of Bio-Fertilizer in Organic Agriculture: A Review. Res. J. Recent 2013, 2, 39–41. [Google Scholar]
- Lee, K.H.; Jose, S. Soil Respiration and Microbial Biomass in a Pecan—Cotton Alley Cropping System in Southern USA. Agrofor. Syst. 2003, 58, 45–54. [Google Scholar] [CrossRef]
- Kremer, R.J.; Kussman, R.D. Soil Quality in a Pecan-Kura Clover Alley Cropping System in the Midwestern USA. Agrofor. Syst. 2011, 83, 213–223. [Google Scholar] [CrossRef]
- Duchene, O.; Vian, J.F.; Celette, F. Intercropping with Legume for Agroecological Cropping Systems: Complementarity and Facilitation Processes and the Importance of Soil Microorganisms. A Review. Agric. Ecosyst. Environ. 2017, 240, 148–161. [Google Scholar] [CrossRef]
- Pérez, H. Manual Para El Manejo Orgánico Del Nogal Pecanero; Palibrio: Bloomington, IN, USA, 2014; p. 274. [Google Scholar]
- Malik, N.S.A.; Perez, J.L.; Lombardini, L.; Cornacchia, R.; Cisneros-Zevallosb, L.; Braforda, J. Phenolic Compounds and Fatty Acid Composition of Organic and Conventional Grown Pecan Kernels. J. Sci. Food Agric. 2009, 89, 2207–2213. [Google Scholar] [CrossRef]
- FAO. FAOSTAT, Produccion de Nuez Con Cáscara. 2018. Available online: http://www.fao.org/faostat/es/#data/QC (accessed on 21 April 2020).
- SADER. Panorama Agroalimentario 2019. Available online: https://federacion-anech.org/2019/11/14/atlas-agroalimentario-2019/ (accessed on 21 April 2020).
- Mungai, N.W.; Motavalli, P.P.; Kremer, R.J. Soil Organic Carbon and Nitrogen Fractions in Temperate Alley Cropping Systems. Commun. Soil Sci. Plant Anal. 2006, 37, 977–992. [Google Scholar] [CrossRef]
- Bai, C.; Reilly, C.C.; Wood, B.W. Nickel Deficiency Disrupts Metabolism of Ureides, Amino Acids, and Organic Acids of Young Pecan Foliage. Plant Physiol. 2006, 140, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Brown, V.; Braun de Torrez, E.; McCracken, G. Crop Pests Eaten by Bats in Organic Pecan Orchards. Crop Prot. 2015, 67, 66–71. [Google Scholar] [CrossRef]
- INIFAP; CONABIO. Instituto Nacional de Investigaciones Forestales y Agropecuarias (INIFAP)—Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO), (1995). “Edafología”. Escalas 1:250000 y 1:1000000. México. Available online: http://www.conabio.gob.mx/informacion/gis/ (accessed on 2 November 2020).
- INEGI. Prontuario de Información Geográfica Municipal, Allende, Coahuila de Zaragoza. Available online: http://www3.inegi.org.mx/contenidos/app/mexicocifras/datos_geograficos/05/05030.pdf (accessed on 2 April 2020).
- INIFAP. Tecnología de Producción En Nogal Pecanero, Prmera; Salinas, H., Quiroga, H., Tijerina, A., Figueroa, U., Eds.; INIFAP-SAGARPA: Matamoros, Mexico, 2002; p. 221. [Google Scholar]
- Jackson, L. Análisis Químico de Suelos, 4th ed.; Beltrán, M., Ed.; Omega: Barcelona, Spain, 1982; p. 662. [Google Scholar]
- Bremner, J.M. Inorganic Forms of Nitrogen in Soil. In Methods of Soil Analysis; Black, C.A., Ed.; Crop Science Society of America: Ames, IA, USA, 1965; pp. 1179–1237. [Google Scholar] [CrossRef]
- Olsen, S.; Sommers, L. Phosphurus. In Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1982; pp. 404–430. [Google Scholar]
- Thomas, G. Exchangeable Cations. In Methods of Soil Analysis; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 159–165. [Google Scholar]
- Walkley, A.; Black, I. An Examination of Degtjareff Method for Determining Soil Organic Matter and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–37. [Google Scholar] [CrossRef]
- Anderson, D.; Schoenau, J. Soli Humus Fractions. In Soli Sampling Methods of Analysis; Carter, M., Ed.; CRC Press: Boca Ratón, FL, USA, 1993; pp. 391–395. [Google Scholar]
- Dębska, B.; Długosz, J.; Piotrowska-Długosz, A.; Banach-Szott, M. The Impact of a Bio-Fertilizer on the Soil Organic Matter Status and Carbon Sequestration—Results from a Field-Scale Study. J. Soils Sediments 2016, 16, 2335–2343. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.Y.; Bowman, A.; Oates, A. Oxidizible Organic Carbon Fractions and Soil Quality Changes in an Oxic Paleustalf under Different Pasture Leys. Soil Sci. 2001, 166, 61–67. [Google Scholar] [CrossRef]
- Brookes, P.; Landman, A.; Pruden, G.; Jenkinson, D. Chloroform Fumigation and the Release of Soil Nitrogen; a Rapid Direct Extraction Method to Measure Microbial Biomasa Nitrogen in Soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ramsey, P.W.; Morris, S.; Paul, E.A. Glomalin, an Arbuscular-Mycorrhizal Fungal Soil Protein, Responds to Land-Use Change. Plant Soil 2003, 253, 293–299. [Google Scholar] [CrossRef]
- Guerrero, P.; Quintero, R.; Espinoza, V.; Benedicto, G.; Sanchez, M. Respiration of CO2 as an Indicator of Microbial Activity in Organic Fertilizers of Lupinus. Terra Latinoam. 2012, 30, 355–362. [Google Scholar]
- García, A.; Rivero, C. Evaluación Del Carbono Microbiano y La Respiración Basal En Respuesta a La Aplicación de Lodo Papelero En Los Suelos de La Cuenca Del Lago de Valencia. Rev. Fac. Agron. 2008, 34, 215–229. [Google Scholar]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The Effects of Long Term Nitrogen Deposition on Extracellular Enzyme Activity in an Acer saccharum Forest Soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G. Oxidation of Non-Phenolic Substrates. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Tabatabai, M. Soil Enzymes. In Methods of Soil Analysis; Weavwe, R., Ed.; American Society of Agronomy: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Illumina. 16S Metagenomic Sequencing Library Preparation, Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System. Available online: https://emea.illumina.com/content/dam/illuminasupport/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (accessed on 20 February 2019).
- Illumina. Nextera XT DNA Library Prep Kit Reference Guide. Available online: https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/samplepreps_nextera/nextera-xt/nextera-xt-library-prep-reference-guide-15031942-05.pdf (accessed on 20 February 2019).
- Caporaso, J.G.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; Huttley, G.A.; Kelley, S.T.; Knights, D.; McDonald, D.; Muegge, B.D.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-De la Peña, C.; Garduño-Niño, E.; Vaca-Paniagua, F.; Díaz-Velásquez, C.; Barrows, C.; Gomez-Gil, B.; Valenzuela-Núñez, L. Comparison of the Fecal Bacterial Microbiota Composition between Wild and Captive Bolson Tortoises (Gopherus flavomarginatus). Herpetol. Conserv. Biol. 2019, 14, 587–600. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Compton, J.E.; Watrud, L.S.; Porteous, L.A.; DeGrood, S. Response of Soil Microbial Biomass and Community Composition to Chronic Nitrogen Additions at Harvard Forest. For. Ecol. Manag. 2004, 196, 143–158. [Google Scholar] [CrossRef]
- Idowu, O.J.; Sanogo, S.; Brewer, C.E. Short-Term Impacts of Pecan Waste By-Products on Soil Quality in Texturally Different Arid Soils. Commun. Soil Sci. Plant Anal. 2017, 48, 1781–1791. [Google Scholar] [CrossRef]
- Kruse, J.; Abraham, M.; Amelung, W.; Baum, C.; Bol, R.; Kühn, O.; Lewandowski, H.; Niederberger, J.; Oelmann, Y.; Rüger, C.; et al. Innovative Methods in Soil Phosphorus Research: A Review. J. Plant Nutr. Soil Sci. 2015, 178, 43–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benbi, D.K.; Brar, K.; Toor, A.S.; Singh, P.; Singh, H. Soil Carbon Pools under Poplar-Based Agroforestry, Rice-Wheat, and Maize-Wheat Cropping Systems in Semi-Arid India. Nutr. Cycl. Agroecosyst. 2012, 92, 107–118. [Google Scholar] [CrossRef]
- Mäder, P.; Fließbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil Fertility and Biodiversity in Organic Farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef] [Green Version]
- Culman, S.W.; DuPont, S.T.; Glover, J.D.; Buckley, D.H.; Fick, G.W.; Ferris, H.; Crews, T.E. Long-Term Impacts of High-Input Annual Cropping and Unfertilized Perennial Grass Production on Soil Properties and Belowground Food Webs in Kansas, USA. Agric. Ecosyst. Environ. 2010, 137, 13–24. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; Zhang, Y.; Fan, T. Long-Term Effect of Manure and Fertilizer on Soil Organic Carbon Pools in Dryland Farming in Northwest China. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Brookes, P.; Cayuela, M.L.; Contin, M.; De Nobili, M.; Kemmitt, S.J.; Mondini, C. The Mineralisation of Fresh and Humified Soil Organic Matter by the Soil Microbial Biomass. Waste Manag. 2008, 28, 716–722. [Google Scholar] [CrossRef]
- de Souza, G.P.; de Figueiredo, C.C.; de Sousa, D.M.G. Relationships between Labile Soil Organic Carbon Fractions under Different Soil Management Systems. Sci. Agric. 2016, 73, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Martínez, E.; Fuentes, J.P.; Acevedo, E. Carbono Orgánico y Propiedades Del Suelo. Revista de la Ciencia del Suelo y Nutrición Vegetal 2008, 8, 68–96. [Google Scholar] [CrossRef]
- Ginebra, M.; Rodríguez, M.; Calero, B.; Ponce de León, D.; Font, L. The Labile Carbon as Indicator of Changes in Two Soils under Different Uses. Cultiv. Trop. 2015, 36, 64–70. [Google Scholar]
- Dignac, M.F.; Derrien, D.; Barré, P.; Barot, S.; Cécillon, L.; Chenu, C.; Chevallier, T.; Freschet, G.T.; Garnier, P.; Guenet, B.; et al. Increasing Soil Carbon Storage: Mechanisms, Effects of Agricultural Practices and Proxies. A Review. Agron. Sustain. Dev. 2017, 37, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Lejon, D.; Sebastia, J.; Lamy, I.; Chaussod, R.; Ranjard, L. Relationships between Soil Organic Status and Microbial Community Density and Genetic Structure in Two Agricultural Soils Submitted to Various Types of Organic Management. Microb. Ecol. 2007, 53, 650–663. [Google Scholar] [CrossRef]
- Abril, A.; Noe, L.; Filippini, M.F. Manejo de Enmiendas Para Restaurar La Materia Orgánica Del Suelo En Oasis de Regadío de Mendoza, Argentina. Rev. Investig. Agropecu. 2014, 40, 83–91. [Google Scholar]
- Vásquez, J.R.; Macías, F.; Menjivar, J.C. Respiración Del Suelo Según Su Uso y Su Relación Con Algunas Formas de Carbono En El Departamento Del Magdalena, Colombia. Bioagro 2013, 25, 175–180. [Google Scholar]
- Álvaro-Fuentes, J.; Cantero-Martínez, C.; López, M.; Arrúe, J. Fijación de Carbono y Reducción de Emisiones de CO2. In Aspectos Agronómicos y Medioambientales de la Agricultura de Conservación; González, E.K., Ordóñez, R., Gil, J.A., Eds.; Ministerio de Medio Ambiente y Medio Rural y Marino: Zaragoza, Spain, 2010; pp. 89–96. [Google Scholar]
- Jinbo, Z.; Changchun, S.; Wenyan, Y. Land Use Effects on the Distribution of Labile Organic Carbon Fractions through Soil Profiles. Soil Sci. Soc. Am. J. 2006, 70, 660–667. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; De Courcelles, V.D.R.; Singh, K.; et al. The Knowns, Known Unknowns and Unknowns of Sequestration of Soil Organic Carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Báez-Pérez, A.; González-Chávez, M.C.Á.; Etchevers-Barra, J.D.; Prat, C.; Hidalgo-Moreno, C. Glomalina y Secuestro de Carbono En Tepetates Cultivados. Agrociencia 2010, 44, 517–529. [Google Scholar]
- Treseder, K.K.; Turner, K.M. Glomalin in Ecosystems. Soil Sci. Soc. Am. J. 2007, 71, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Otto, B.; Schlosser, D.; Reisser, W. First Description of a Laccase-like Enzyme in Soil Algae. Arch. Microbiol. 2010, 192, 759–768. [Google Scholar] [CrossRef]
- Rivera-Hoyos, C.M.; Morales-Álvarez, E.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; RodrÍguez-Vázquez, R.; Delgado-Boada, J.M. Fungal Laccases. Fungal Biol. Rev. 2013, 27, 67–82. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Estiarte, M. Changes in Soil Enzymes Related to C and N Cycle and in Soil C and N Content under Prolonged Warming and Drought in a Mediterranean Shrubland. Appl. Soil Ecol. 2008, 39, 223–235. [Google Scholar] [CrossRef]
- Aragón, R.; Sardans, J.; Peñuelas, J. Soil Enzymes Associated with Carbon and Nitrogen Cycling in Invaded and Native Secondary Forests of Northwestern Argentina. Plant Soil 2014, 384, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Dong, W.; Dai, X.; Schaeffer, S.; Yang, F.; Radosevich, M.; Xu, L.; Liu, X.; Sun, X. Responses of Absolute and Specific Soil Enzyme Activities to Long Term Additions of Organic and Mineral Fertilizer. Sci. Total Environ. 2015, 536, 59–67. [Google Scholar] [CrossRef]
- Kotroczó, Z.; Veres, Z.; Fekete, I.; Krakomperger, Z.; Tóth, J.A.; Lajtha, K.; Tóthmérész, B. Soil Enzyme Activity in Response to Long-Term Organic Matter Manipulation. Soil Biol. Biochem. 2014, 70, 237–243. [Google Scholar] [CrossRef]
- Vigdis, T.; Øvreås, L. Microbial Diversity, Life Strategies, and Adaptation to Life in Extreme Soils. In Microbiology of Extreme Soils; Dion, P., Shekhar, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 16, pp. 15–43. [Google Scholar] [CrossRef]
- Carbonetto, B.; Rascovan, N.; Álvarez, R.; Mentaberry, A.; Vázquez, M.P. Structure, Composition and Metagenomic Profile of Soil Microbiomes Associated to Agricultural Land Use and Tillage Systems in Argentine Pampas. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef]
- Pan, Y.; Cassman, N.; de Hollander, M.; Mendes, L.W.; Korevaar, H.; Geerts, R.H.E.M.; van Veen, J.A.; Kuramae, E.E. Impact of Long-Term N, P, K, and NPK Fertilization on the Composition and Potential Functions of the Bacterial Community in Grassland Soil. FEMS Microbiol. Ecol. 2014, 90, 195–205. [Google Scholar] [CrossRef]
- Rabbi, S.M.F.; Daniel, H.; Lockwood, P.V.; Macdonald, C.; Pereg, L.; Tighe, M.; Wilson, B.R.; Young, I.M. Physical Soil Architectural Traits Are Functionally Linked to Carbon Decomposition and Bacterial Diversity. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Goodfellow, M.; Williams, S.T. Ecology of Actinomycetes. Annu. Rev. Microbiol. 1983, 37, 189–216. [Google Scholar] [CrossRef]
- Ozdemir-Kocak, F.; Isik, K.; Saricaoglu, S.; Saygin, H.; Inan-Bektas, K.; Cetin, D.; Guven, K.; Sahin, N. Kribbella sindirgiensis sp. Nov. Isolated from Soil. Arch. Microbiol. 2017, 199, 1399–1407. [Google Scholar] [CrossRef]
- Liu, Y.R.; Delgado-Baquerizo, M.; Yang, Z.; Feng, J.; Zhu, J.; Huang, Q. Microbial Taxonomic and Functional Attributes Consistently Predict Soil CO2 Emissions across Contrasting Croplands. Sci. Total Environ. 2020, 702, 1–8. [Google Scholar] [CrossRef]
- Devos, D.P. Gemmata obscuriglobus. Curr. Biol. 2013, 23, 705–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuerst, J.A.; Lee, K.-C.; Butler, M.K. Gemmata. Bergey’s Man. Syst. Archaea Bact. 2015, 1–5. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, X.; Wang, J.; Li, X.; Guo, Q.; Yu, Z.; Yang, T.; Zhang, H. Long-Term No-Tillage and Different Residue Amounts Alter Soil Microbial Community Composition and Increase the Risk of Maize Root Rot in Northeast China. Soil Tillage Res. 2020, 196, 104452. [Google Scholar] [CrossRef]
- Faria, M.; Bordin, N.; Kizina, J.; Harder, J.; Devos, D.; Lage, O.M. Planctomycetes Attached to Algal Surfaces: Insight into Their Genomes. Genomics 2018, 110, 231–238. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Q.; Zhou, J.; Wei, Q. Pyrosequencing Technology Reveals the Impact of Different Manure Doses on the Bacterial Community in Apple Rhizosphere Soil. Appl. Soil Ecol. 2014, 78, 28–36. [Google Scholar] [CrossRef]
- Talamantes, D.; Biabini, N.; Dang, H.; Abdoun, K.; Berlemont, R. Natural Diversity of Cellulases, Xylanases, and Chitinases in Bacteria. Biotechnol. Biofuels 2016, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kovaleva, O.L.; Elcheninov, A.G.; Kublanov, I.V.; Bonch-Osmolovskaya, E. Tepidisphaera. Bergey’s Man. Syst. Archaea Bact. 2019, 1, 1–5. [Google Scholar] [CrossRef]
- Sørensen, S.; Ronen, Z.; Aamand, J. Isolation from Agricultural Soil and Characterization of a Sphingomonas sp. Able to Mineralize the Phenylurea Herbicide Isoproturon. Society 2001, 67, 5403–5409. [Google Scholar] [CrossRef] [Green Version]
- Chater, K.F.; Biró, S.; Lee, K.J.; Palmer, T.; Schrempf, H. The Complex Extracellular Biology of Streptomyces. FEMS Microbiol. Rev. 2010, 34, 171–198. [Google Scholar] [CrossRef] [Green Version]
- Seipke, R.F.; Kaltenpoth, M.; Hutchings, M.I. Streptomyces as Symbionts: An Emerging and Widespread Theme? FEMS Microbiol. Rev. 2012, 36, 862–876. [Google Scholar] [CrossRef] [Green Version]
- de Orué Lucana, D.O.; Schaa, T.; Schrempf, H. The Novel Extracellular Streptomyces reticuli Haem-Binding Protein HbpS Influences the Production of the Catalase-Peroxidase CpeB. Microbiology 2004, 150, 2575–2585. [Google Scholar] [CrossRef] [Green Version]
- Tuncer, M.; Kuru, A.; Isikli, M.; Sahin, N.; Çelenk, F.G. Optimization of Extracellular Endoxylanase, Endoglucanase and Peroxidase Production by Streptomyces sp. F2621 Isolated in Turkey. J. Appl. Microbiol. 2004, 97, 783–791. [Google Scholar] [CrossRef]
- Wu, S.; Li, Y.; Wang, P.; Zhong, L.; Qiu, L.; Chen, J. Shifts of Microbial Community Structure in Soils of a Photovoltaic Plant Observed Using Tag-Encoded Pyrosequencing of 16S RRNA. Appl. Microbiol. Biotechnol. 2016, 100, 3735–3745. [Google Scholar] [CrossRef]
- Pascual, J.; Huber, K.J.; Foesel, B.U.; Overmann, J. Stenotrophobacter. Bergey’s Man. Syst. Archaea Bact. 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Li, W.H.; Liu, Q.Z.; Chen, P. Effect of Long-Term Continuous Cropping of Strawberry on Soil Bacterial Community Structure and Diversity. J. Integr. Agric. 2018, 17, 2570–2582. [Google Scholar] [CrossRef]
- Marschner, P.; Kandeler, E.; Marschner, B. Structure and Function of the Soil Microbial Community in a Long-Term Fertilizer Experiment. Soil Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
- Yu, C.; Hu, X.M.; Deng, W.; Li, Y.; Xiong, C.; Ye, C.H.; Han, G.M.; Li, X. Changes in Soil Microbial Community Structure and Functional Diversity in the Rhizosphere Surrounding Mulberry Subjected to Long-Term Fertilization. Appl. Soil Ecol. 2015, 86, 30–40. [Google Scholar] [CrossRef]
- Simpson, A.J.; Song, G.; Smith, E.; Lam, B.; Novotny, E.H.; Hayes, M.H.B. Unraveling the Structural Components of Soil Humin by Use of Solution-State Nuclear Magnetic Resonance Spectroscopy. Environ. Sci. Technol. 2007, 41, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.R.; Männistö, M.K.; Bromberg, Y.; Häggblom, M.M. Comparative Genomic and Physiological Analysis Provides Insights into the Role of Acidobacteria in Organic Carbon Utilization in Arctic Tundra Soils. FEMS Microbiol. Ecol. 2012, 82, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Eichorst, S.A.; Trojan, D.; Roux, S.; Herbold, C.; Rattei, T.; Woebken, D. Genomic Insights into the Acidobacteria Reveal Strategies for Their Success in Terrestrial Environments. Environ. Microbiol. 2018, 20, 1041–1063. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Kong, W.; Wang, F.; Long, X.E.; Guo, C.; Yue, L.; Yao, H.; Dong, X. Desert and Steppe Soils Exhibit Lower Autotrophic Microbial Abundance but Higher Atmospheric CO2 Fixation Capacity than Meadow Soils. Soil Biol. Biochem. 2018, 127, 230–238. [Google Scholar] [CrossRef]
- Miltner, A.; Richnow, H.H.; Kopinke, F.D.; Kästner, M. Assimilation of CO2 by Soil Microorganisms and Transformation into Soil Organic Matter. Org. Geochem. 2004, 35, 1015–1024. [Google Scholar] [CrossRef]
- Videmšek, U.; Hagn, A.; Suhadolc, M.; Radl, V.; Knicker, H.; Schloter, M.; Vodnik, D. Abundance and Diversity of CO2-Fixing Bacteria in Grassland Soils Close to Natural Carbon Dioxide Springs. Microb. Ecol. 2009, 58, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arcand, M.M.; Helgason, B.L.; Lemke, R.L. Microbial Crop Residue Decomposition Dynamics in Organic and Conventionally Managed Soils. Appl. Soil Ecol. 2016, 107, 347–359. [Google Scholar] [CrossRef]
- Singh, G.; Bhattacharyya, R.; Das, T.K.; Sharma, A.R.; Ghosh, A.; Das, S.; Jha, P. Crop Rotation and Residue Management Effects on Soil Enzyme Activities, Glomalin and Aggregate Stability under Zero Tillage in the Indo-Gangetic Plains. Soil Tillage Res. 2018, 184, 291–300. [Google Scholar] [CrossRef]




| Variable | Organic | Conventional | t | df | p |
|---|---|---|---|---|---|
| pH | 8.1 ± 0.09 | 8.1 ± 0.05 | 1 | 3 | 0.391 |
| Nitrogen% | 0.050 ± 0.004 | 0.043 ± 0.003 | 3.13 | 6 | 0.020 |
| Phosphorous mg kg−1 | 16.3 ± 5.80 | 5.6 ± 1.00 | 4.901 | 6 | 0.003 |
| Potassium mg L−1 | 0.52 ± 0.31 | 0.31 ±0.19 | 2.06 | 3.14 | 0.127 * |
| Organic Matter% | 2.97 ± 0.31 | 3.11 ± 0.11 | −812 | 6 | 0.448 |
| Organic Carbon% | 1.91 ± 0.04 | 1.72 ± 0.02 | 7.071 | 6 | 0.000 |
| Relation C/N% | 38.6 ± 3.10 | 40.5 ± 2.00 | −1.116 | 6 | 0.307 |
| Very labile fraction C g kg−1 | 12.30 ± 1.35 | 12.80 ± 1.59 | −0.522 | 6 | 0.620 |
| Labile fraction C g kg−1 | 7.57 ± 0.70 | 10.65 ± 0.45 | −6.392 | 6 | 0.001 |
| Less labile fraction C g kg−1 | 18.67 ± 3.17 | 18.50 ± 0.91 | −0.068 | 6 | 0.948 |
| Recalcitrant fraction C g kg−1 | 4.94 ± 3.03 | 2.43 ± 0.49 | 1.822 | 3.58 | 0.151 * |
| Mineralization of C mg CO2 g−1 | 176.0 ± 29.30 | 278.5 ± 64.80 | −2.781 | 6 | 0.032 |
| Easily Extractable Glomalin mg g−1 | 0.5 ± 0.00 | 0.8 ± 0.00 | −9.076 | 6 | 0.000 |
| Microbial biomass carbon µg C g−1 | 751.1 ± 73.70 | 77.0 ± 0.00 | 45.195 | 3 | 0.000 |
| Colony forming units g−1 | 516,000 ± 323,777 | 1,200,777 ± 701,683 | −1.516 | 6 | 0.180 |
| Humic acids mg C kg−1 | 1657.4 ± 91.90 | 2618 ± 181.80 | −10.464 | 6 | 0.000 |
| Fulvic acids mg C kg−1 | 8671.4 ± 144.20 | 9632.2 ± 144.10 | −8.497 | 6 | 0.000 |
| Humins mg C kg−1 | 14,316.3 ± 221.80 | 7350 ± 166.40 | 50.229 | 6 | 0.000 |
| Peroxidase µmol g−1 h−1 | 4.34 ± 0.71 | 4.50 ± 0.16 | −0.472 | 6 | 0.653 |
| Polyphenol oxidase µmol g−1 h−1 | 6.41 ± 0.53 | 7.23 ± 0.57 | −2.021 | 6 | 0.090 |
| Lacasse µmol g−1 h−1 | 0.02 ± 0.01 | 0.18 ± 0.00 | −13 | 6 | 0.000 |
| B-glucosidase mg PNP g−1 | 145.1 ± 7.20 | 510.6 ± 19.5 | −42.287 | 6 | 0.000 |
| B-galactosidase mg PNP g−1 | 24.2 ± 9.50 | 54.4 ± 1.00 | −4.608 | 3.03 | 0.019 * |
| Sample | Total | Assembled | Discarded | CD | QS | BS | OTUs |
|---|---|---|---|---|---|---|---|
| Org 1 | 278,980 | 68,366 | 210,608 | 568 | 67,798 | 27,154 | 6014 |
| Org 2 | 225,583 | 52,984 | 172,599 | 486 | 52,498 | 22,087 | 5594 |
| Org 3 | 187,477 | 57,859 | 129,607 | 610 | 57,249 | 34,945 | 6377 |
| Mean | 230,680 | 59,736 | 170,938 | 555 | 59,182 | 28,062 | 5995 |
| Conv 1 | 212,217 | 51,586 | 160,618 | 523 | 51,063 | 33,292 | 6022 |
| Conv 2 | 272,201 | 83,983 | 188,207 | 870 | 83,113 | 53,264 | 8653 |
| Conv 3 | 318,199 | 94,434 | 223,744 | 842 | 93,592 | 59,769 | 9135 |
| Mean | 267,539 | 76,668 | 190,856 | 745 | 75,923 | 48,775 | 7937 |
| Genera | Relative Abundance% | |
|---|---|---|
| Organic | Conventional | |
| Tepidisphaera | 3.8 | 7.1 * |
| GQ396871 | 3.9 | 3.7 |
| Sphingomonas | 2.6 | 3.2 * |
| Gemmata | 4.0 * | 3.1 |
| Dongia | 3.1 * | 2.1 |
| FJ478799 | 4.2 | 2.0 |
| Microvirga | 0.9 | 1.9 * |
| GQ263023 | 1.4 | 1.8 |
| Sphingosinicella | 0.5 | 1.7 * |
| Streptomyces | 1.5 | 1.6 * |
| Rhizomicrobium | 1.1 | 1.3 * |
| EU335288 | 0.9 | 1.2 |
| AF370880 | 1.6 | 1.2 |
| FJ479444 | 0.7 | 1.2 |
| Stenotrophobacter | 0.9 | 1.2 * |
| EF125410 | 0.3 | 1.2 |
| EU669599 | 0.3 | 1.1 |
| Pseudolabrys | 1.2 * | 1.1 |
| Zavarzinella | 1.7 * | 1.1 |
| EU335161 | 1.1 | 1.0 |
| Catelliglobosispora | 1.2 * | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Rodríguez, A.; Nava-Reyna, E.; Trejo-Calzada, R.; García-De la Peña, C.; Arreola-Ávila, J.G.; Collavino, M.M.; Vaca-Paniagua, F.; Díaz-Velásquez, C.; Constante-García, V. Effect of Organic and Conventional Systems Used to Grow Pecan Trees on Diversity of Soil Microbiota. Diversity 2020, 12, 436. https://doi.org/10.3390/d12110436
Cabrera-Rodríguez A, Nava-Reyna E, Trejo-Calzada R, García-De la Peña C, Arreola-Ávila JG, Collavino MM, Vaca-Paniagua F, Díaz-Velásquez C, Constante-García V. Effect of Organic and Conventional Systems Used to Grow Pecan Trees on Diversity of Soil Microbiota. Diversity. 2020; 12(11):436. https://doi.org/10.3390/d12110436
Chicago/Turabian StyleCabrera-Rodríguez, Alejandra, Erika Nava-Reyna, Ricardo Trejo-Calzada, Cristina García-De la Peña, Jesús G. Arreola-Ávila, Mónica M. Collavino, Felipe Vaca-Paniagua, Clara Díaz-Velásquez, and Vicenta Constante-García. 2020. "Effect of Organic and Conventional Systems Used to Grow Pecan Trees on Diversity of Soil Microbiota" Diversity 12, no. 11: 436. https://doi.org/10.3390/d12110436
APA StyleCabrera-Rodríguez, A., Nava-Reyna, E., Trejo-Calzada, R., García-De la Peña, C., Arreola-Ávila, J. G., Collavino, M. M., Vaca-Paniagua, F., Díaz-Velásquez, C., & Constante-García, V. (2020). Effect of Organic and Conventional Systems Used to Grow Pecan Trees on Diversity of Soil Microbiota. Diversity, 12(11), 436. https://doi.org/10.3390/d12110436