Common Patterns and Diverging Trajectories in Primary Succession of Plants in Eastern Alpine Glacier Forelands
Abstract
:1. Introduction
- (1)
- Do common patterns in species composition, plant functional traits, and/or vegetation structure exist in the four glacier forelands studied?
- (2)
- Does vegetation development over time follow analogous successional trajectories or do divergences during succession appear?
- (3)
- If so, is there a particular point in time when trajectories begin to diverge and what might be underlying reasons?
2. Materials and Methods
2.1. Study Areas
2.2. Vegetation Data
- JTF: Total length 2.15 km; elevational difference 330 m; 9 different sample locations (A: 1 year.; B: 7 years.; C: 15 years.; D: 25 years.; E: 55 years.; F: 70 years.; G: 90 years.; H: 120 years.; I: 150 years).
- SBF: Length 2.1 km; elevational difference 615 m; 10 different sample locations (A: 4 years.; B: 5 years.; C: 15 years.; D: 20 years.; E: 40 years.; F: 60 years.; G: 80 years.; H: 110 years.; I: 130 years.; J: 155 years.)
- LSF: Length 1.25 km; elevational difference 260 m; 9 different sample locations (A: 2 years.; B: 4 years.; C: 20 years.; D: 35 years.; E: 55 years.; F: 75 years.; G: 90 years.; H: 120 years.; I: 155 years.)
- GBK: Length 1.3 km; elevational difference 220 m; 8 different sample locations (A: 2 years.; B: 4 years.; C: 15 years.; D: 25–30 years.; E: 55 years.; F: 85 years.; G: 120 years.; H: 155 years.)
- Life-form composition (according to [44]: therophytes (Th, annual herbaceous plants), geophytes (G, plants with tuberous subterranean organs), graminoid hemicryptophytes (H gram, perennial grasses), herbaceous hemicryptophytes (H herb, perennial herbaceous plants), chamaephytes (Ch, woody dwarfshrubs growing less than 0.5 m tall), nanophanerophytes (NaPh, shrubs growing 0.5–2.0 m tall), and macrophanerophytes (MacPh, trees reaching 20–50 m in adult stage), as well as moss and lichens. The life-form classification followed [43].
- Ecological indicator values of vascular plants (according to [45]) were used for an ecological assessment of sample sites. Values vary between 1 and 5 and the following parameters were used: Soil moisture (dry = 1, wet = 5), light (shady = 1, full sun = 5), continentality (oceanic = 1, continental = 5), temperature (alpine/nival = 1, collinean = 5), pH (acidic = 1, basic = 5), soil aeration/texture (compacted = 1, loose, rocky/sandy = 5), soil humus content (humus-free = 1, peaty = 5), and soil nutrients content (oligotrophic = 1, eutrophic = 5).
2.3. Data Analysis
3. Results
3.1. Floristic Aspects, Species Richness Patterns, and Ground Cover Development
3.2. Patterns in Vegetation Structure, Life-Form Composition, and Ecological Plant Traits
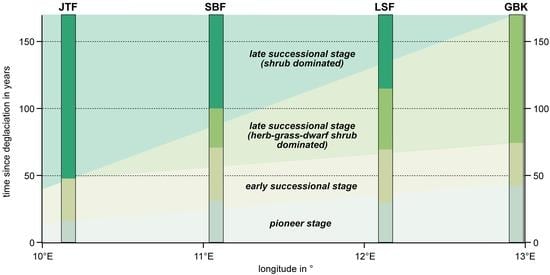
3.3. Successional Stages and Trajectories
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Warming, E. Plantesamfund: Grundtræk af den økologiske Plantegeografi; Philipsens Kjøbenhavn: Kopenhagen, Denmark, 1895; p. 335. [Google Scholar]
- Cowles, H.C. The Ecological Relations of the Vegetation on the Sand Dunes of Lake Michigan; The University of Chicago Press: Chicago, IL, USA, 1899. [Google Scholar]
- Clements, F.E. Plant Succession: An Analysis of the Development of Vegetation; Carnegie Institution of Washington: Washington, DC, USA, 1916; p. 512. [Google Scholar]
- Ellenberg, H. Vegetation Ecology of Central Europe; Cambridge University Press: Cambridge, UK, 1988; p. 731. [Google Scholar]
- Matthews, J.A. The Ecology of Recently-Deglaciated Terrain. A Geoecological Approach to Glacier Forelands and Primary Succession; Cambridge University Press: Cambridge, UK, 1992; p. 386. [Google Scholar]
- Walker, L.R.; Del Moral, R. Primary Succession and Ecosystem Rehabilitation; Cambridge University Press: Cambridge, UK, 2003; p. 442. [Google Scholar]
- Erschbamer, B.; Caccianiga, M.S. Glacier forelands: Lessons of plant population and community development. Prog. Bot. 2017, 78, 259–284. [Google Scholar]
- Paul, F.; Kääb, A.; Maisch, M.; Kellenberger, T.; Haeberli, W. Rapid disintegration of Alpine glaciers observed with satellite data. Geophys. Res. Lett. 2004, 31, L21402. [Google Scholar] [CrossRef] [Green Version]
- Zemp, M.; Haeberli, W.; Hoelzle, M.; Paul, F. Alpine glaciers to disappear within decades? Geophys. Res. Lett. 2006, 33, L13504. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.B.; Seiser, B.; Stocker Waldhuber, M.; Mitterer, C.; Abermann, J. Tracing glacier changes in Austria from the Little Ice Age to the present using a lidar-based high-resolution glacier inventory in Austria. Cryosphere 2015, 9, 753–766. [Google Scholar] [CrossRef] [Green Version]
- Pickett, S.T.A. Space-for-time substitution as an alternative to long-term studies. In Long-Term Studies in Ecology: Approaches and Alternatives; Likens, G.E., Ed.; Springer: New York, NY, USA, 1989; pp. 110–135. [Google Scholar]
- Fischer, A.; Fickert, T.; Schwaizer, G.; Patzelt, G.; Groß, G. Vegetation dynamics in Alpine glacier forelands tackled from space. Nature Sci. Rep. 2019, 9, 13918. [Google Scholar] [CrossRef] [Green Version]
- Walker, L.R.; Wardle, D.A.; Bardgett, R.D.; Clarkson, B.D. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 2010, 98, 725–736. [Google Scholar] [CrossRef]
- Coaz, J. Erste Ansiedlung Phanerogamer Pflanzen auf von Gletschern Verlassenem Boden; Mitteilungen der Naturforschenden Gesellschaft Bern: Bern, Switzerland, 1887; pp. 3–12. [Google Scholar]
- Von Klebelsberg, R. Das Vordringen der Hochgebirgsvegetation in den Tiroler Alpen. Eine alpin-pflanzengeographische Studie. Osterr. botan. Zeitschrift 1913, 63, 177–186. [Google Scholar] [CrossRef]
- Lüdi, W. Beobachtungen über die Besiedlung von Gletschervorfeldern in den Schweizeralpen. Flora oder Allg. Bot. Ztg. 1958, 146, 386–407. [Google Scholar] [CrossRef]
- Pirola, A.; Credaro, V. Changes in the vegetation of a recent glacial moraine in the Bernina group. Annali di Botanica LI 1993, 51, 145–153. [Google Scholar]
- Andreis, C.; Caccianiga, M.; Cerabolini, B. Vegetation and environmental factors during primary succession on glacier forelands. Plant Biosyst. 2001, 135, 295–310. [Google Scholar] [CrossRef]
- Caccianiga, M.; Andreis, C. Pioneer herbaceous vegetation on glacier forelands in the Italian Alps. Phytocoenologia 2004, 34, 55–89. [Google Scholar] [CrossRef]
- Raffl, C.; Mallaun, M.; Mayer, R.; Erschbamer, B. Vegetation succession pattern and diversity changes in a Glacier Valley, Central Alps, Austria. Arct. Antarct. Alp. Res. 2006, 38, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Cannone, N.; Diolaiuti, G.; Guglielmin, M.; Smiraglia, C. Accelerating climate change impacts on alpine glacier forefield ecosystems in the European Alps. Ecol. Appl. 2008, 18, 637–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erschbamer, B.; Niederfriniger Schlag, R.; Winkler, E. Colonization processes on a central alpine glacier foreland. J. Veg. Sci. 2008, 19, 855–862. [Google Scholar] [CrossRef]
- Burga, C.A.; Krüsi, B.; Egli, M.; Wernli, M.; Elsener, S.; Ziefle, M.; Fischer, T.; Mavrisa, M. Plant succession and soil development on the foreland of the Morteratsch glacier (Pontresina, Switzerland): Straight forward or chaotic? Flora 2010, 205, 561–576. [Google Scholar] [CrossRef] [Green Version]
- Loher, T.; Sextl, K.; Grüninger, F.; Fickert, T. Gletscherrückgang und Vegetationsentwicklung im Vorfeld des Schwarzenbergferners (Stubaier Alpen, Tirol) seit dem Ende der Kleinen Eiszeit. Jahrb. des Ver. zum Schutz der Bergwelt 2013, 78, 139–164. [Google Scholar]
- Schumann, K.; Gewolf, S.; Tackenberg, O. Factors affecting primary succession of glacier foreland vegetation in the European Alps. Alp. Bot. 2016, 126, 105–117. [Google Scholar] [CrossRef]
- Fickert, T. Glacier forelands—Unique field laboratories for the study of primary succession of plants. In Glaciers Evolution in a Changing World; Godone, D., Ed.; InTech Open: London, UK, 2017; pp. 125–146. [Google Scholar] [CrossRef] [Green Version]
- Isotta, F.A.; Frei, C.; Weilguni, V.; Perčec Tadić, M.; Lassègues, P.; Rudolf, B.; Pavan, V.; Caccia-mani, C.; Antolini, G.; Ratto, S.M. The climate of daily precipitation in the Alps: Development and analysis of a high-resolution grid dataset from pan-Alpine rain-gauge data. Int. J. Climatol. 2014, 34, 1657–1675. [Google Scholar] [CrossRef]
- Hijmans, R.; Cameron, S.; Parra, J.; Jones, P.; Jarvis, A. Very high resolution interpolated climate surfaces of global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Abermann, J.; Kuhn, M.; Fischer, A. Climatic controls of glacier distribution and glacier changes in Austria. Ann. Glaciol. 2011, 52, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Auer, I.; Böhm, R.; Jurkovic, A.; Lipa, W.; Orlik, A.; Potzmann, R.; Schöner, W.; Ungersböck, M.; Matulla, C.; Briffa, K. HISTALP—Historical instrumental climatological surface time series of the greater Alpine region 1760-2003. Int. J. Climatol. 2007, 27, 17–46. [Google Scholar] [CrossRef]
- Ehlers, J.; Gibbard, P.L. Quaternary glaciations—extent and chronology: Part I: Europe. In Developments in Quaternary Sciences; Elsevier: Amsterdam, The Netherlands, 2004; p. 488. [Google Scholar]
- Ehlers, J.; Gibbard, P.L.; Hughes, P.D. Quaternary glaciations—extent and chronology: A closer look. In Developments in Quaternary Sciences; Elsevier: Amsterdam, The Netherlands, 2011; p. 1126. [Google Scholar]
- Engler, A. Pflanzen-Formationen und die Pflanzengeographische Gliederung der Alpenkette Erläutert an der Alpenanlage des Neuen Königlichen Botanischen Gartens zu Dahlem-Steglitz bei Berlin, mit 2 Orientierungskarten; Wilhelm Engelmann: Leipzig, Germany, 1903; p. 96. [Google Scholar]
- Merxmüller, H. Untersuchungen zur Sippengliederung und Arealbildungen in den Alpen. In Jahrbuch des Vereins zum Schutz der Alpenpflanzen und -tiere; Verein zum Schutz der Alpenpflanzen und -tiere: Munich, Germany, 1952; Volume 17, pp. 96–133. [Google Scholar]
- Stehlik, I. Nunataks and peripheral refugia for alpine plants during Quaternary glaciation in the middle part of the Alps. Bot. Helv. 2000, 110, 25–30. [Google Scholar]
- Parisod, C. Postglacial recolonisation of plants in the western Alps of Switzerland. Bot. Helv. 2008, 118, 1–12. [Google Scholar] [CrossRef]
- Schneeweiss, G.M.; Schönswetter, P. A re-appraisal of nunatak survival in arctic-alpine phylogeography. Mol. Ecol. 2011, 20, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Aeschimann, D.; Rasolofo, N.; Theurillat, J.-P. Analyse de la Flore des Alpes. 1: Historique et Biodiversité. Candollea 2011, 66, 27–55. [Google Scholar] [CrossRef]
- ZAMG Climate Data for Austria 1971–2000. Available online: https://www.zamg.ac.at/fix/klima/oe71-00/klima2000/klimadaten_oesterreich_1971_frame1.htm (accessed on 6 April 2020).
- Wikipedia Alpenrelief 01. Available online: https://de.wikipedia.org/wiki/Alpen#/media/File:Alpenrelief_01.jpg (accessed on 5 April 2020).
- Böhm, R.; Schöner, W.; Auer, I.; Hynek, B.; Kroisleitner, C.; Weyss, G. Gletscher im Klimawandel: Vom Eis der Polargebiete zum Goldbergkees in den Hohen Tauern; Zentralanstalt für Meteorologie und Geodynamik: Wien, Austria, 2007; p. 111. [Google Scholar]
- Damm, B. Gletscher-, Landschafts- und Klimaentwicklung in der Rieserfernergruppe (Tirol) seit dem Spätglazial. Göttinger Geogr. Abh. 1996, 104, 186. [Google Scholar]
- Fischer, M.A.; Adler, W.; Oswald, K. Exkursionsflora für Österreich, Liechtenstein und Südtirol; Land Oberösterreich, OÖ Landesmuseum: Linz, Austria, 2005; p. 1380. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: Oxford, UK, 1934; p. 632. [Google Scholar]
- Landolt, E.; Bäumler, B.; Erhardt, A.; Hegg, O.; Klötzli, F.A.; Lämmler, W.; Nobis, M.; Rudmann-Maurer, K.; Schweingruber, F.H.; Theurillat, J.-P.; et al. Flora Indicativa: Ökologische Zeigerwerte und Biologische Kennzeichen zur Flora der Schweiz und der Alpen; Haupt Verlag AG: Bern, Switzerland, 2010; p. 376. [Google Scholar]
- Ter Braak, C.J.F.; Barendregt, L.G. Weighted averaging of species indicator values: Its efficiency in environmental calibration. Math. Biosci. 1986, 78, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, M.R.F. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Biometris: Wageningen, The Netherlands, 2002; p. 499. [Google Scholar]
- Sørensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. K. Dan. Vidensk. Selsk. 1948, 5, 1–34. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical ecology. In Developments in Environmental Modelling; Elsevier: Amsterdam, The Netherlands, 2012; Volume 24, p. 1006. [Google Scholar]
- Becker, T.; Dierschke, H. Primärsukzession im Gletschervorfeld des Obersulzbachkees (Hohe Tauern, Österreich), eine Zeitreihe über fast 150 Jahre. Tuexenia 2005, 25, 111–139. [Google Scholar]
- Timoshok, E.E.; Timoshok, E.N.; Gureyeva, I.I.; Skorokhodov, S.N. Primary Successions of Vegetation on the Young Moraines in the Severo-Chuiskiy Center of Glaciation (Central Altai). Contemp. Probl. Ecol. 2020, 13, 36–47. [Google Scholar] [CrossRef]
- Robbins, J.A.; Matthews, J.A. Regional Variation in Successional Trajectories and Rates of Vegetation Change on Glacier Forelands in South-Central Norway. Arct. Antarct. Alp. Res. 2010, 42, 351–361. [Google Scholar] [CrossRef]
- Zimmer, A.; Meneses, R.I.; Rabatel, A.; Soruco, A.; Dangles, O.; Anthelme, F. Time lag between glacial retreat and upward migration alters tropical alpine communities. Perspect. Plant Ecol. Evol. Syst. 2018, 30, 89–102. [Google Scholar] [CrossRef]
- Glausen, T.G.; Tanner, L.H. Successional trends and processes on a glacial foreland in Southern Iceland studied by repeated species counts. Ecol. Process. 2019, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, M.E.; Freppaz, M.; Filippa, G.; Zanini, E. Vegetation influence on soil formation rate in a proglacial chronosequence (Lys Glacier, NW Italian Alps). Catena 2014, 113, 122–137. [Google Scholar] [CrossRef]
- D’Amico, M.E.; Freppaz, M.; Zanini, E.; Bonifacio, E. Primary vegetation succession and the serpentine syndrome: The proglacial area of the Verra Grande glacier, North-Western Italian Alps. Plant Soil 2017, 415, 283–298. [Google Scholar] [CrossRef]
- Fickert, T.; Grüninger, F. High-speed colonization of bare ground—Permanent plot studies on primary succession of plants in recently deglaciated glacier forelands. Land Degrad. Dev. 2018, 29, 2668–2680. [Google Scholar] [CrossRef]
- Stöcklin, J.; Bäumler, E. Seed rain, seedling establishment and clonal growth strategies on a glacier foreland. J. Veg. Sci. 1996, 7, 45–56. [Google Scholar] [CrossRef]
- Tackenberg, O.; Stöcklin, J. Wind dispersal of alpine plant species: A comparison with lowland species. J. Veg. Sci. 2008, 19, 109–118. [Google Scholar] [CrossRef]
- Haselwandter, K.; Hofmann, A.; Holzmann, H.P.; Read, D.J. Availability of nitrogen and phosphorus in the nival zone of the Alps. Oecologia 1983, 57, 266–269. [Google Scholar] [CrossRef]
- Heer, C.; Körner, C. High elevation pioneer plants are sensitive to mineral nutrient addition. Basic Appl. Ecol. 2002, 3, 39–47. [Google Scholar] [CrossRef]
- Hiltbrunner, E.; Schwikowski, M.; Körner, C. Inorganic nitrogen storage in alpine snow pack in the Central Alps (Switzerland). Atmos. Environ. 2005, 39, 2249–2259. [Google Scholar] [CrossRef]
- Göransson, H.; Welc, M.; Bünemann, E.K.; Christl, I.; Venterink, H.O. Nitrogen and phosphorus availability at early stages of soil development in the Damma glacier forefield, Switzerland; implications for establishment of N2-fixing plants. Plant Soil 2016, 404, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Nemergut, D.R.; Anderson, S.P.; Cleveland, C.C.; Martin, A.P.; Miller, A.E.; Seimon, A.; Schmidt, S.K. Microbial Community Succession in an Unvegetated, Recently Deglaciated Soil. Microb. Ecol. 2007, 53, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Nascimbene, J.; Mayrhofer, H.; Dainese, M.; Bilovitz, P.O. Assembly patterns of soil-dwelling lichens after glacier retreat in the European Alps. J. Biogeogr. 2017, 44, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.A.; Singarayer, J.S.; Anesio, A.M. Microbial community dynamics in the forefield of glaciers. Proc. R. Soc. B 2014, 281, 20140882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darcy, J.L.; Schmidt, S.K.; Knelman, J.E.; Cleveland, C.C.; Castle, S.C.; Nemergut, D.R. Phosphorous, not nitrogen, limits plants and microbial primary producers following glacial retreat. Sci. Adv. 2018, 4, eaaq0942. [Google Scholar] [CrossRef] [Green Version]
- Fickert, T.; Grüninger, F.; Damm, B. Klebelsberg revisited: Did primary succession of plants in glacier forelands a century ago differ from today? Alp. Bot. 2017, 127, 17–29. [Google Scholar] [CrossRef]
- Braun-Blanquet, J.; Jenny, H. Vegetationsentwicklung und Bodenbildung in der alpinen Stufe der Zentralalpen. Denkschr. der Schweiz. Nat. Ges. 1926, 63, 183–343. [Google Scholar]
- Englisch, T.; Valachovic, M.; Mucona, L.; Grabherr, G.; Ellmauer, T. Thlaspietea rotundifolii. In Die Pflanzengesellschaften Österreichs Teil. II.; Grabherr, G., Mucina, L., Eds.; Gustav Fischer Verla: Jena, Germany, 1993; pp. 276–342. [Google Scholar]
- Gobbi, M.; De Bernardi, F.; Pelfini, M.; Rossaro, B.; Brandmayr, P. Epigean Arthropod Succession along a 154-year Glacier Foreland Chronosequence in the Forni Valley (Central Italian Alps). Arct. Antarct. Alp. Res. 2006, 38, 357–362. [Google Scholar] [CrossRef]
- Körner, C.; Paulsen, J. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Proske, H.; Granika, K.; Mudri-Raninger, R. Die Landschaft der beiden Täler. In Zwei Alpentäler im Klimawandel; Auer, I., Prettenthaler, F., Böhm, R., Proske, H., Eds.; Innsbruck University Press: Innsbruck, Austria, 2010; pp. 83–118. [Google Scholar]








| Key Figures | JTF | SBF | LSF | GBK |
|---|---|---|---|---|
| latitude/longitude of glacier terminus at time of sampling | 46°52′ N; 10°09′ E | 47°02′ N; 11°00′ E | 46°55′ N; 12°08′ E | 47°02′ N; 12°58′ E |
| latitude/longitude of LIA terminal moraine | 46°53′ N;10°10′ E | 47°01′ N; 11°04′ E | 46°56′ N; 12°08′ E | 47°03′ N; 12°58′ E |
| exposure | N | W | N & NW | N & NE |
| approx. temperature within glacier foreland | −0.5 °C to −2.5 °C | 0 °C to −3.6 °C | 0 °C to −1.7 °C | −0.3 °C to −1.6 °C |
| approx. precipitation within glacier foreland | 1500 mm | 1300 mm | 1300 mm | 1600 mm |
| geology | metamorphic rocks (gneiss, amphibolite) | metamorphic rocks (gneiss, mica-schist) | granitoide rocks | granitoide rocks |
| elevation highest samples | 2450 m a.s.l. | 2780 m a.s.l. | 2600 m a.s.l. | 2400 m a.s.l. |
| elevation of lowest sample | 2120 m a.s.l. | 2165 m a.s.l. | 2340 m a.s.l. | 2180 m a.s.l. |
| horizontal extent of the chronosequence | 2150 m | 2100 m | 1250 m | 1300 m |
| vertical extent of the chronosequence | 330 m | 615 m | 260 m | 220 m |
| number of sample locations per chronosequence | 9 | 10 | 9 | 8 |
| Sørensen Similarity Index | JTF-SBF | JTF-LSF | JTF-GBK | SBF-LSF | SBF-GBK | LSF-GBK |
|---|---|---|---|---|---|---|
| Entire species inventory | 0.62 | 0.58 | 0.49 | 0.69 | 0.65 | 0.66 |
| Pioneer stage | 0.70 | 0.64 | 0.52 | 0.70 | 0.64 | 0.71 |
| Early successional stage | 0.54 | 0.53 | 0.48 | 0.55 | 0.63 | 0.58 |
| Late successional stage | 0.63 | 0.55 | 0.46 | 0.68 | 0.61 | 0.62 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fickert, T. Common Patterns and Diverging Trajectories in Primary Succession of Plants in Eastern Alpine Glacier Forelands. Diversity 2020, 12, 191. https://doi.org/10.3390/d12050191
Fickert T. Common Patterns and Diverging Trajectories in Primary Succession of Plants in Eastern Alpine Glacier Forelands. Diversity. 2020; 12(5):191. https://doi.org/10.3390/d12050191
Chicago/Turabian StyleFickert, Thomas. 2020. "Common Patterns and Diverging Trajectories in Primary Succession of Plants in Eastern Alpine Glacier Forelands" Diversity 12, no. 5: 191. https://doi.org/10.3390/d12050191
APA StyleFickert, T. (2020). Common Patterns and Diverging Trajectories in Primary Succession of Plants in Eastern Alpine Glacier Forelands. Diversity, 12(5), 191. https://doi.org/10.3390/d12050191