Biological Control of Salvinia molesta (D.S. Mitchell) Drives Aquatic Ecosystem Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
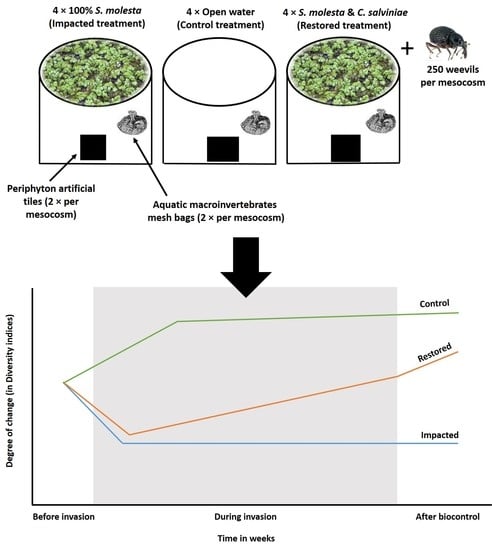
2.2. Experimental Design
2.3. Data Collection
2.3.1. Water Physicochemical Variables
2.3.2. Biological Data
Epilithic Algae Assemblage
Phytoplankton and Periphyton Algal Biomass
Aquatic Macroinvertebrates
2.4. Data Analysis
2.4.1. Physicochemical Variables
2.4.2. Epilithic Algae and Aquatic Macroinvertebrate Diversity Patterns and Response Ratios
2.4.3. Epilithic Algae and Aquatic Macroinvertebrate Community Assemblage Structure
2.4.4. Biological Diversity Responses to Physicochemical Variables
3. Results
3.1. Physicochemical Variables
3.2. Epilithic Algae and Aquatic Macroinvertebrate Diversity Patterns
3.3. Biodiversity Indices Mean Response Ratios
3.4. Epilithic Algae and Aquatic Macroinvertebrate Assemblage Structure
3.5. Biological Diversity Response to Physicochemical Variables
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bakker, E.S.; Sarneel, J.M.; Gulati, R.D.; Liu, Z.; van Donk, E. Restoring macrophyte diversity in shallow temperate lakes: Biotic versus abiotic constraints. Hydrobiologia 2013, 710, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.P. The impact and control of alien aquatic vegetation in South African aquatic ecosystems. Afr. J. Aquat. Sci. 2003, 28, 19–24. [Google Scholar] [CrossRef]
- Hill, M.P.; Coetzee, J. The biological control of aquatic weeds in South Africa: Current status and future challenges. Bothalia 2017, 47, 1–12. [Google Scholar] [CrossRef]
- De Tezanos Pinto, P.; Allende, L.; O’Farrell, I. Influence of free-floating plants on the structure of a natural phytoplankton assemblage: An experimental approach. J. Plankton Res. 2007, 29, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2002, 290, 521–523. [Google Scholar] [CrossRef]
- Corbin, J.D.; D’Antonio, C.M. Abundance and productivity mediate invader effects on nitrogen dynamics in a California grassland. Ecosphere 2011, 2. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; Raghu, S.; Sheppard, A.W.; Schaffner, U. Quantifying the social and economic benefits of the biological control of invasive alien plants in natural ecosystems. Curr. Opin. Insect Sci. 2020, 38, 1–5. [Google Scholar] [CrossRef]
- Mcfadyen, R.E.C. Successes in biological control of weeds. Proc. X Int. Symp. Biol. Control Weeds 2000, 14, 3–14. [Google Scholar]
- Van Driesche, R.G.; Pratt, P.D.; Huber, J.; Hough-Goldstein, J.; Newman, R.; Sands, D.; Causton, C.E.; Cuda, J.; Miller, R.; Ding, J.; et al. Classical biological control for the protection of natural ecosystems. Biol. Control 2010, 54, S2–S33. [Google Scholar] [CrossRef]
- McConnachie, A.J.; De Wit, M.P.; Hill, M.P.; Byrne, M.J. Economic evaluation of the successful biological control of Azolla filiculoides in South Africa. Biol. Control 2003, 28, 25–32. [Google Scholar] [CrossRef]
- Fraser, G.C.G.; Hill, M.P.; Martin, J.A. Economic evaluation of water loss saving due to the biological control of water hyacinth at New Year’s Dam, Eastern Cape province, South Africa. Afr. J. Aquat. Sci. 2016, 41, 227–234. [Google Scholar] [CrossRef]
- Arp, R.; Fraser, G.; Hill, M. Quantifying the water savings benefit of water hyacinth (Eichhornia crassipes) control in the Vaalharts Irrigation Scheme. Water SA 2017, 43, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Coetzee, A.J.A.; Hill, M.P.; Byrne, M.J.; Bownes, A. A review of the biological control programmes on Eichhornia crassipes (C. Mart) Solms (Pontederiaceae), Salvinia molesta D. S. Mitch. (Salviniaceae), Pistia stratiotes L. (Araceae), Myriophyllum aquaticum (Vell.) Verdc. (Haloragaceae) and Azolla filiculoides Lam. (Azollaceae) in South Africa. Afr. Entomol. 2011, 19, 451–468. [Google Scholar]
- Cuda, J.P.; Charudattan, R.; Grodowitz, M.J.; Newman, R.M.; Shearer, J.F.; Tamayo, M.L.; Villegas, B. Recent advances in biological control of submersed aquatic weeds. J. Aquat. Plant Manag. 2008, 46, 15–32. [Google Scholar]
- Maseko, Z.; Coetzee, J.A.; Hill, M.P. Effect of shade and eutrophication on the biological control of Salvinia molesta (Salviniaceae) by the weevil Cyrtobagous salviniae (Coleoptera: Erirhinidae). Austral Entomol. 2019, 58, 595–601. [Google Scholar] [CrossRef]
- Martin, G.D.; Coetzee, J.A.; Weyl, P.S.R.; Parkinson, M.C.; Hill, M.P. Biological control of Salvinia molesta in South Africa revisited. Biol. Control 2018, 125, 74–80. [Google Scholar] [CrossRef]
- Schaffner, U.; Hill, M.; Dudley, T.; D’Antonio, C. Post-release monitoring in classical biological control of weeds: Assessing impact and testing pre-release hypotheses. Curr. Opin. Insect Sci. 2020, 38, 99–106. [Google Scholar] [CrossRef]
- Hulme, P.E. Beyond control: Wider implications for the management of biological invasions. J. Appl. Ecol. 2006, 43, 835–847. [Google Scholar] [CrossRef]
- Underwood, A.J. Beyond BACI: The detection of environmental impacts on populations in the real, but variable, world. J. Exp. Mar. Bio. Ecol. 1992, 161, 145–178. [Google Scholar] [CrossRef]
- Pryke, J.S.; Samways, M.J. Recovery of invertebrate diversity in a rehabilitated city landscape mosaic in the heart of a biodiversity hotspot. Landsc. Urban Plan. 2009, 93, 54–62. [Google Scholar] [CrossRef]
- Magoba, R.N.; Samways, M.J. Recovery of benthic macroinvertebrate and adult dragonfly assemblages in response to large scale removal of riparian invasive alien trees. J. Insect Conserv. 2010, 14, 627–636. [Google Scholar] [CrossRef]
- Samways, M.J.; Sharratt, N.J.; Simaika, J.P. Effect of alien riparian vegetation and its removal on a highly endemic river macroinvertebrate community. Biol. Invasions 2011, 13, 1305–1324. [Google Scholar] [CrossRef]
- Modiba, R.V.; Joseph, G.S.; Seymour, C.L.; Fouché, P.; Foord, S.H. Restoration of riparian systems through clearing of invasive plant species improves functional diversity of Odonate assemblages. Biol. Conserv. 2017, 214, 46–54. [Google Scholar] [CrossRef]
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The role of benthic invertebrate species in freshwater ecosystems: Zoobenthic species influence energy flows and nutrient cycling. Bioscience 1999, 49, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Kietzka, G.J.; Pryke, J.S.; Samways, M.J. Landscape ecological networks are successful in supporting a diverse dragonfly assemblage. Insect Conserv. Diver. 2015, 8, 229–237. [Google Scholar] [CrossRef]
- Liboriussen, L.; Jeppesen, E. Structure, biomass, production and depth distribution of periphyton on artificial substratum in shallow lakes with contrasting nutrient concentrations. Freshw. Biol. 2006, 51, 95–109. [Google Scholar] [CrossRef]
- Coetzee, J.A.; Jones, R.W.; Hill, M.P. Water hyacinth, Eichhornia crassipes (Pontederiaceae), reduces benthic macroinvertebrate diversity in a protected subtropical lake in South Africa. Biodivers. Conserv. 2014, 23, 1319–1330. [Google Scholar] [CrossRef]
- Stiers, I.; Crohain, N.; Josens, G.; Triest, L. Impact of three aquatic invasive species on native plants and macroinvertebrates in temperate ponds. Biol. Invasions 2011, 13, 2715–2726. [Google Scholar] [CrossRef]
- Thomas, P.; Room, P. Towards biological control of salvinia in Papua New Guines. Proc. Int. Symp. Biol. Control Weeds 1985, 567–574. [Google Scholar]
- Nelson, L.S.; Skogerboe, J.G.; Getsinger, K.D. Herbicide evaluation against giant salvinia. J. Aquat. Plant Manag. 2001, 39, 48–53. [Google Scholar]
- Julien, M.H.; Hill, M.P.; Tipping, P.W. Salvinia molesta D. S. Mitchell (Salviniaceae). In Biological Control of Tropical Weeds Using Arthropods; Muniappan, R., Reddy, G.V.P., Raman, A., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 378–407. ISBN 9780511576348. [Google Scholar]
- Hennecke, B.; Postle, L. The key to success: An investigation into oviposition of the salvinia weevil in cool climate regions. In Proceedings of the 15th Australia Weeds Conference, Weed Management Society of South Australia, Adelaide, South Australia, 24–28 September 2006; pp. 780–783. [Google Scholar]
- Room, P.M.; Thomas, P.A. Nitrogen, phosphorus and potassium in Salvinia molesta Mitchell in the field: Effects of weather, insect damage, fertilizers and age. Aquat. Bot. 1986, 24, 213–232. [Google Scholar] [CrossRef]
- Flores, D.; Wendel, L. Proposed Field Release of the Salvinia Weevil, Cyrtobagous Salviniae Calder and Sands (Curculionidae: Coleoptera), A Host-Specific Biological Control Agent of Giant Salvinia, Salvinia Molesta D.S. Mitchell (Salviniaceae: Polypodiophyta) A Federal Noxious Weed; USD/APHIS/PPQ/CPHST/Mission Plant Protection Center (MPPC): Edinburg, TX, USA, 2001. [Google Scholar]
- Oliver, J.D. A review of the biology of giant salvinia (Salvinia molesta Mitchell). J. Aquat. Plant Manag. 1993, 31, 227–231. [Google Scholar]
- Doeleman, J.A. Biological Control of Salvinia Molesta in Sri Lanka: An Assessment of Costs and Benefits; No 1–No 12; Australian Center for International Agricultural Research: Canberra, Australia, 1989; p. 14. ISBN 1949511978. [Google Scholar]
- Sullivan, P.; Postle, L. Salvinia Biological Control Field Guide; Department of Trade and Investment, Regional Infrastructure and Services: Sydney, Australia, 2012; ISBN 9781742562520. [Google Scholar]
- Kelly, M.G.; Cazaubon, A.; Coring, E.; Dell’Uomo, A.; Ector, L.; Goldsmith, B.; Guasch, H.; Hurlimann, J.; Jarlman, A.; Kawecka, B.; et al. Recommendations for the routine sampling of diatoms for water quality assessments in Europe. J. Appl. Phycol. 1998, 10, 215–224. [Google Scholar] [CrossRef]
- Taylor, J.C.; Harding, W.R.; Archibald, C.G.M. A Methods Manual for the Collection, Preparation and Analysis of Diatom Samples Version 1.0; Water Research Commission: Pretoria, South Africa, 2007; pp. 1–49. [Google Scholar]
- LeGresley, M.; McDermott, G. Counting chamber methods for quantitative phytoplankton analysis-haemocytometer, Palmer-Maloney cell and Sedgewick-Rafter cell. In Manuals and Guides: Microscopic and Molecular Methods for Quantitative Phytoplankton Analysis, 55th ed.; Karlson, B., Cusack, C., Bresnan, E., Eds.; United Nations Educational, Scientific and Cultural Organisation: Paris, France, 2000; pp. 25–30. [Google Scholar]
- John, D.; Whitton, B.; Brook, A.J. (Eds.) The Freshwater Algal Flora of the British Isles An Identification Guide to Freshwater and Terrestrial Algae; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Van Vuuren, J.; Taylor, J.; van Ginkel, C.; Gerber, A. A Guide for the Identification of Microscopic Algae in South African Freshwaters; North West University and Department of Water Affairs and Forestry: Pretoria, South Africa, 2006; ISBN 0621354716. [Google Scholar]
- Taylor, J.C.; Harding, W.R.; Archibald, G.M. An Illustrated Guide to Some Common Diatom Species from South Africa; Water Research Commission: Pretoria, South Africa, 2007; ISBN 09263373. [Google Scholar]
- Andersen, P.; Throndsen, J. Estimating cell numbers. In Monographs on Oceanographic Methodology: Manual on Harmful Marine Microalgae; Hallegraeff, G., Anderson, D., Cembella, A., Eds.; United Nations Educational, Scientific and Cultural Organisation: Paris, France, 2003; pp. 99–130. ISBN 9231039482. [Google Scholar]
- Holm-hansen, O.; Riemann, B. Chlorophyll-a determination: Improvements in Methodology. Oikos 1978, 30, 438–447. [Google Scholar] [CrossRef]
- Dalu, T.; Froneman, P.W.; Richoux, N.B.; Dalu, T.; Froneman, P.W. Phytoplankton community diversity along a river- estuary continuum. Trans. R. Soc. South Afr. 2014, 69, 107–116. [Google Scholar] [CrossRef]
- Lorenzen, C. Determination for chlorophyll and pheophytin pigments. Plant Physiol. 1967, 12, 343–346. [Google Scholar]
- Daemen, E.A.M.J. Comparison of methods for the determination of chlorophyll in estuarine sediments. Neth. J. Sea Res. 1986, 20, 21–28. [Google Scholar] [CrossRef]
- Dickens, C.W.S.; Graham, P.M. The South African scoring system (SASS) version 5 rapid bioassessment method for rivers. Afr. J. Aquat. Sci. 2002, 27, 1–10. [Google Scholar] [CrossRef]
- Booth, A.J.; Kadye, W.T.; Vu, T.; Wright, M. Rapid colonisation of artificial substrates by macroinvertebrates in a South African lentic environment. Afr. J. Aquat. Sci. 2013, 38, 175–183. [Google Scholar] [CrossRef]
- Thirion, C. A new biomonitoring protocol to determine the ecological health of impoundments using artificial substrates. Afr. J. Aquat. Sci. 2000, 25, 123–133. [Google Scholar] [CrossRef]
- Day, J.A.; de Moor, I.J. Guides to the Freshwater Invertebrates of Southern Africa. Volume 5: Non-Arthropods; Water Research Commission: Pretoria, South Africa, 2002. [Google Scholar]
- Day, J.A.; de Moor, I.J.; Stewart, B.A.; Louw, A. Guides to the Freshwater Invertebrates of Southern Africa. Volume 3: Crustacea II; Water Research Commission: Pretoria, South Africa, 2001. [Google Scholar]
- Day, J.A.; de Moor, I.J. Guides to the Freshwater Invertebrates of Southern Africa. Volume 6: Arachnida and Mollusca; Water Research Commission: Pretoria, South Africa, 2002. [Google Scholar]
- Day, J.A.; Stewart, B.; de Moor, I.J.; Louw, A. Guides to the Freshwater Invertebrates of Southern Africa. Volume 4: Crustacea III; Water Research Commission: Pretoria, South Africa, 2001. [Google Scholar]
- Gerber, A.; Gabriel, M. Aquatic Invertebrates of South African Rivers: Field Guide; Department of Water Affairs: Pretoria, South Africa, 2002. [Google Scholar]
- Bunn, A.; Korpela, M. R Core Team R: A language and environment for statistical computing. R Found. Stat. Comput. 2016, 2, 2630. [Google Scholar]
- Oksanen, J. Vegan: Ecological diversity. R Proj. 2013, 1, 12. [Google Scholar]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Benayas, J.M.R.; Newton, A.C.; Diaz, A.; Bullock, J.M. Enhancement of biodiversity and ecosystem services by ecological restoration: A meta-analysis. Science 2009, 325, 1121–1124. [Google Scholar] [CrossRef]
- Bates, D.; Machler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kenward, M.G.; Roger, J.H. An improved approximation to the precision of fixed effects from restricted maximum likelihood. Comput. Stat. Data Anal. 2009, 53, 2583–2595. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1978, 6, 65–70. [Google Scholar]
- Zuur, A.; Leno, E.; Walker, N.; Saveliev, A.; Smith, G. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2006; ISBN 9780387874579. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User manual/tutorial. PRIMER-E: Plymouth, UK, 2006; Available online: https://www.researchgate.net/profile/Saeed_Ebrahimnezhad_Darzi/post/looking_for_PDF_of_Primer_v6_User_Manual_Tutorial/attachment/59d6515879197b80779a9e3b/AS%3A506949617238016%401497877615728/download/ClarkeGorley+2006+PrimerV6UserManual.pdf (accessed on 1 December 2017).
- Anderson, M.; Willis, T. Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual and Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Cummins, K.; Klug, M. Feeding ecology of stream invertebrates. Annu. Rev. Ecol. Syst. 1979, 10, 147–172. [Google Scholar] [CrossRef]
- Palmer, C.G.; Maart, B.; Palmer, A.R.; O’Keeffe, J.H. An assessment of macroinvertebrate functional feeding groups as water quality indicators in the Buffalo River, eastern Cape Province, South Africa. Hydrobiologia 1996, 318, 153–164. [Google Scholar] [CrossRef]
- Ingram, B.; Hawking, J.; Shiel, R. Aquatic life in Freshwater Ponds: A Guide to the Identification and Ecology of Life in Aquaticulture Ponds and Farm Dams in South-Eastern Australia; Co-Operative Research Center for Freshwater Ecology: Albury, Australia, 1997. [Google Scholar]
- Walker, K.; Madden, C.; Williams, C.; Fricker, S.; Geddes, M.; Goonan, P.; Kokkinn, M.; McEvoy, P.; Shiel, R.; Tsymbal, V. Freshwater Inverterbrates. In Natural History of the Riverland and Murraylands; Jennings, J., Ed.; Royal Society of South Australia Inc.: Adelaide, Australia, 2009; p. 416. ISBN 9780959662795. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Random and Mixed Effects. In Modern Applied Statistics with S; Venables, W., Ripley, B.D., Eds.; Statistics and Computing: New York, NY, USA, 2002; pp. 271–300. [Google Scholar]
- Masifwa, W.F.; Twongo, T.; Denny, P. The impact of water hyacinth, Eichhornia crassipes (Mart) solms on the abundance and diversity of aquatic macroinvertebrates along the shores of northern Lake Victoria, Uganda. Hydrobiologia 2001, 452, 79–88. [Google Scholar] [CrossRef]
- Brendonck, L.; Maes, J.; Rommens, W.; Dekeza, N.; Nhiwatiwa, T.; Barson, M.; Callebaut, V.; Phiri, C.; Moreau, K.; Gratwicke, B.; et al. The impact of water hyacinth (Eichhornia crassipes) in a eutrophic subtropical impoundment (Lake Chivero, Zimbabwe). II. Species diversity. Arch. für Hydrobiol. 2003, 158, 389–405. [Google Scholar] [CrossRef]
- Midgley, J.M.; Hill, M.P.; Villet, M.H. The effect of water hyacinth, Eichhornia crassipes (Martius) Solms-Laubach (Pontederiaceae), on benthic biodiversity in two impoundments on the New Year’s River, South Africa. Afr. J. Aquat. Sci. 2006, 31, 25–30. [Google Scholar] [CrossRef]
- Chamier, J.; Schachtschneider, K.; le Maitre, D.C.; Ashton, P.J.; van Wilgen, B.W. Impacts of invasive alien plants on water quality, with particular emphasis on South Africa. Water SA 2012, 38, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Adams, S.; Hill, W.; Peterson, M.; Ryon, M.; Smith, J.; Stewart, A. Assessing recovery in a stream ecosystem: Applying multiple chemical and biological endpoints. Ecol. Appl. 2002, 12, 1510–1527. [Google Scholar] [CrossRef]
- Muotka, T.; Laasonen, P. Ecosystem recovery in restored headwater streams. J. Appl. Ecol. 2002, 39, 145–156. [Google Scholar] [CrossRef]
- Kettenring, K.M.; Adams, C.R. Lessons learned from invasive plant control experiments: A systematic review and meta-analysis. J. Appl. Ecol. 2011, 48, 970–979. [Google Scholar] [CrossRef]
- Prior, K.M.; Adams, D.C.; Klepzig, K.D.; Hulcr, J. When does invasive species removal lead to ecological recovery? Implications for management success. Biol. Invasions 2018, 20, 267–283. [Google Scholar] [CrossRef]
- Richardson, J.; Feuchtmayr, H.; Miller, C.; Hunter, P.D.; Maberly, S.C.; Carvalho, L. Response of cyanobacteria and phytoplankton abundance to warming, extreme rainfall events and nutrient enrichment. Glob. Chang. Biol. 2019, 25, 1–16. [Google Scholar]
- Strange, E.F.; Hill, J.M.; Coetzee, J.A. Evidence for a new regime shift between floating and submerged invasive plant dominance in South Africa. Hydrobiologia 2018, 817, 349–362. [Google Scholar] [CrossRef]
- Strange, E.F.; Landi, P.; Hill, J.M.; Coetzee, J.A. Modeling top-down and bottom-up drivers of a regime shift in invasive aquatic plant stable states. Front. Plant Sci. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Turunen, J.; Louhi, P.; Mykrä, H.; Aroviita, J.; Putkonen, E.; Huusko, A.; Muotka, T. Combined effects of local habitat, anthropogenic stress, and dispersal on stream ecosystems: A mesocosm experiment. Ecol. Appl. 2018, 28, 1606–1615. [Google Scholar] [CrossRef]
- Benton, T.G.; Solan, M.; Travis, J.M.J.; Sait, S.M. Microcosm experiments can inform global ecological problems. Trends Ecol. Evol. 2007, 22, 516–521. [Google Scholar] [CrossRef]






| Physicochemical Variables | Treatments (df = 2, 111) | Invasion Phases (df = 2, 111) | Treatment × Phase (df = 4, 111) | |||
|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| pH | 2.36 | 0.099 | 6.46 | <0.01 | 6.14 | <0.001 |
| Conductivity (µS) | 1.88 | 0.158 | 1.23 | 0.296 | 0.253 | 0.907 |
| Total Dissolved Solids (ppm) | 0.87 | 0.423 | 1.36 | 0.262 | 0.12 | 0.979 |
| Salinity (ppm) | 2.67 | 0.074 | 0.81 | 0.499 | 0.16 | 0.957 |
| Dissolved Oxygen (mg/L) | 21.74 | <0.001 | 10.39 | <0.001 | 3.15 | 0.017 |
| Water temperature (°C) | 2.44 | 0.092 | 31.27 | <0.001 | 0.06 | 0.993 |
| Water Clarity (cm) | 27.034 | <0.001 | 3.05 | 0.05 | 4.88 | 0.001 |
| NO3 (mg/L) | 20.23 | <0.001 | 2.06 | 0.133 | 2.19 | 0.074 |
| NH4 (mg/L) | 3.49 | 0.03 | 0.97 | 0.383 | 0.32 | 0.863 |
| P (mg/L) | 9.12 | <0.001 | 1.32 | 0.271 | 1.05 | 0.383 |
| Phytoplankton biomass (mg/m3) | 6.03 | 0.003 | 5.49 | 0.005 | 1.61 | 0.178 |
| Periphyton biomass (mg/m3) | 36.52 | <0.001 | 11.45 | <0.001 | 12.27 | <0.001 |
| Fixed Effects | N | S | J | H | ||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| Epilithic algae | ||||||||
| Treatment | 0.59 | 0.56 | 0.57 | 0.57 | 1.32 | 0.29 | 0.33 | 0.72 |
| Invasion phase | 7.01 | <0.01 | 2.90 | 0.05 | 0.08 | 0.91 | 1.01 | 0.37 |
| Treatment × Invasion phase | 1.07 | 0.38 | 1.12 | 0.35 | 1.13 | 0.35 | 1.65 | 0.17 |
| R2marginal | 0.21 | - | 0.15 | - | 0.07 | - | 0.21 | - |
| R2conditional | 0.23 | - | 0.15 | - | 0.18 | - | 0.23 | - |
| Aquatic macroinvertebrates | ||||||||
| Treatment | 4.78 | <0.05 | 24.24 | <0.001 | 3.42 | <0.05 | 22.11 | <0.001 |
| Invasion phase | 4.14 | <0.05 | 5.92 | <0.01 | 5.48 | <0.01 | 7.02 | 0.001 |
| Treatment × Invasion phase | 7.62 | <0.001 | 8.44 | <0.001 | 2.98 | <0.05 | 4.47 | <0.01 |
| R2marginal | 0.39 | - | 0.63 | - | 0.17 | - | 0.55 | - |
| R2conditional | 0.46 | - | 0.63 | - | 0.17 | - | 0.56 | - |
| Diversity Indices | Predictors | Estimates | SE | t | p | AdjR2 | df | F | p |
|---|---|---|---|---|---|---|---|---|---|
| Epilithic Algae | |||||||||
| lnN | Intercept | 31.047 | 7.008 | 4.430 | <0.0001 | 0.308 | 7, 100 | 7.801 | <0.0001 |
| Collector-gathers | 0.260 | 0.140 | 1.852 | 0.067 | |||||
| Cover | −0.543 | 0.095 | −5.730 | <0.0001 | |||||
| pH | −10.114 | 3.311 | −3.055 | 0.003 | |||||
| Water temperature | −1.990 | 1.076 | −1.850 | 0.067 | |||||
| [DO] | 1.354 | 0.896 | 1.512 | 0.134 | |||||
| [NH4] | −9.915 | 6.857 | −1.446 | 0.151 | |||||
| lnS | Intercept | 9.789 | 2.102 | 4.658 | <0.0001 | 0.209 | 5, 102 | 6.646 | <0.0001 |
| Cover | −0.125 | 0.028 | −4.549 | <0.0001 | |||||
| pH | −2.230 | 0.896 | −2.490 | 0.014 | |||||
| Water temperature | −0.724 | 0.312 | −2.320 | 0.022 | |||||
| [NH4] | −4.679 | 2.054 | −2.278 | 0.248 | |||||
| [P] | −0.151 | 0.106 | −1.430 | 0.156 | |||||
| J | Intercept | 0.642 | 0.034 | 18.677 | <0.0001 | 0.027 | 2, 105 | 2.529 | 0.085 |
| Cover | 0.016 | 0.011 | 1.511 | 0.133 | |||||
| [NH4] | −1.511 | 0.878 | −1.721 | 0.088 | |||||
| lnH | Intercept | 4.806 | 2.086 | 2.304 | 0.023 | 0.044 | 3, 104 | 2.659 | 0.05 |
| pH | −1.404 | 0.959 | −1.464 | 0.146 | |||||
| [NH4] | −4.737 | 2.391 | −1.981 | 0.050 | |||||
| [P] | −0.206 | 0.123 | −1.671 | 0.098 |
| Diversity Indices | Predictors | Estimates | SE | t | p | AdjR2 | df | F | p |
|---|---|---|---|---|---|---|---|---|---|
| Aquatic Macroinvertebrates | |||||||||
| N | Intercept | −7.341 | 3.608 | −2.035 | 0.044 | 0.204 | 4, 115 | 8.617 | <0.0001 |
| Cover | −0.122 | 0.046 | −2.667 | 0.009 | |||||
| Phytoplankton biomass | 0.226 | 0.115 | 1.963 | 0.052 | |||||
| pH | 6.192 | 1.707 | 3.628 | 0.0004 | |||||
| [DO] | −0.881 | 0.433 | −2.032 | 0.045 | |||||
| S | Intercept | −39.085 | 13.11 | −2.981 | 0.004 | 0.444 | 4, 115 | 24.74 | <0.0001 |
| Cover | −1.030 | 0.157 | −6.547 | <0.0001 | |||||
| Phytoplankton biomass | 1.059 | 0.393 | 2.692 | 0.008 | |||||
| pH | 16.194 | 5.518 | 2.935 | 0.004 | |||||
| Water temperature | 4.957 | 1.745 | 2.841 | 0.005 | |||||
| J | Intercept | 0.068 | 0.224 | 0.301 | 0.764 | 0.060 | 3, 116 | 3.52 | 0.017 |
| Water clarity | 0.049 | 0.021 | 2.322 | 0.022 | |||||
| Phytoplankton biomass | 0.028 | 0.017 | 1.607 | 0.111 | |||||
| Water temperature | 0.153 | 0.070 | 2.190 | 0.031 | |||||
| H | Intercept | −3.902 | 1.547 | −2.523 | 0.013 | 0.410 | 4, 115 | 21.64 | <0.0001 |
| Cover | −0.111 | 0.019 | −5.993 | <0.0001 | |||||
| Phytoplankton biomass | 0.096 | 0.046 | 2.060 | 0.042 | |||||
| pH | 1.708 | 0.651 | 2.624 | 0.01 | |||||
| Water temperature | 0.629 | 0.209 | 3.102 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motitsoe, S.N.; Coetzee, J.A.; Hill, J.M.; Hill, M.P. Biological Control of Salvinia molesta (D.S. Mitchell) Drives Aquatic Ecosystem Recovery. Diversity 2020, 12, 204. https://doi.org/10.3390/d12050204
Motitsoe SN, Coetzee JA, Hill JM, Hill MP. Biological Control of Salvinia molesta (D.S. Mitchell) Drives Aquatic Ecosystem Recovery. Diversity. 2020; 12(5):204. https://doi.org/10.3390/d12050204
Chicago/Turabian StyleMotitsoe, Samuel N., Julie A. Coetzee, Jaclyn M. Hill, and Martin P. Hill. 2020. "Biological Control of Salvinia molesta (D.S. Mitchell) Drives Aquatic Ecosystem Recovery" Diversity 12, no. 5: 204. https://doi.org/10.3390/d12050204
APA StyleMotitsoe, S. N., Coetzee, J. A., Hill, J. M., & Hill, M. P. (2020). Biological Control of Salvinia molesta (D.S. Mitchell) Drives Aquatic Ecosystem Recovery. Diversity, 12(5), 204. https://doi.org/10.3390/d12050204