A Mediterranean Monk Seal Pup on the Apulian Coast (Southern Italy): Sign of an Ongoing Recolonisation?
Abstract
:1. Introduction
2. Materials and Methods
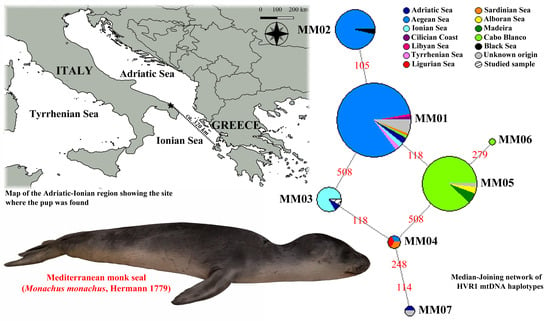
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scheel, D.M.; Slater, G.J.; Kolokotronis, S.O.; Potter, C.W.; Rotstein, D.S.; Tsangaras, K.; Greenwood, A.D.; Helgen, K.M. Biogeography and taxonomy of extinct and endangered monk seals illuminated by ancient DNA and skull morphology. Zookeys 2014, 409, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Karamanlidis, A.A.; Dendrinos, P. Monachus monachus. IUCN Red List Threat. Species 2015; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2015. [Google Scholar] [CrossRef]
- Karamanlidis, A.A.; Dendrinos, P.; de Larrinoa, P.F.; Gücü, A.C.; Johnson, W.M.; Kiraç, C.O.; Pires, R. The Mediterranean monk seal Monachus monachus: Status, biology, threats, and conservation priorities. Mamm. Rev. 2016, 46, 92–105. [Google Scholar] [CrossRef]
- Johnson, W.M. Monk seals in post-classical history. The role of the Mediterranean monk seal (Monachus monachus) in European history and culture, from the fall of Rome to the 20th century. In Mededelingen No. 39; Netherlands Commission for International Nature Protection: Leiden, The Netherlands, 2004; pp. 1–91. [Google Scholar]
- Johnson, W.M.; Lavigne, D.M. Monk seals in antiquity. The Mediterranean monk seal (Monachus monachus) in ancient history and literature. In Mededelingen No. 35; Netherlands Commission for International Nature Protection: Leiden, The Netherlands, 1999. [Google Scholar]
- Pastor, T.; Aguilar, A. Reproductive cycle of the female Mediterranean monk seal in the Western Sahara. Mar. Mamm. Sci. 2003, 19, 318–330. [Google Scholar] [CrossRef]
- Gucu, A.C.; Gucu, G.; Orek, H. Habitat use and preliminary demographic evaluation of the critically endangered Mediterranean monk seal (Monachus monachus) in the Cilician Basin (Eastern Mediterranean). Biol. Conserv. 2004, 116, 417–431. [Google Scholar] [CrossRef]
- Karamanlidis, A.A.; Paravas, V.; Trillmich, F.; Dendrinos, P. First observations of parturition and postpartum behavior in the Mediterranean monk seal (Monachus monachus) in the eastern Mediterranean. Aquat. Mamm. 2010, 36, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Gazo, M.; Layna, J.F.; Aparicio, F.; Cedenilla, M.A.; González, L.M.; Aguilar, A. Pupping season, perinatal sex ratio and natality rates of the Mediterranean monk seal (Monachus monachus) from the Cabo Blanco colony. J. Zool. 1999, 249, 393–401. [Google Scholar] [CrossRef]
- Stringer, C.B.; Finlayson, J.C.; Barton, R.N.E.; Fernández-Jalvo, Y.; Cáceres, I.; Sabin, R.C.; Rhodes, E.J.; Currant, A.P.; Rodríguez-Vidal, J.; Giles-Pacheco, F.; et al. Neanderthal exploitation of marine mammals in Gibraltar. Proc. Natl. Acad. Sci. USA 2008, 105, 14319–14324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Pérez, J.V.; Pérez Ripoll, M.; Jordá Pardo, J.F.; Álvarez-Fernández, E.; Maestro González, A.; Aura Tortosa, J.E. Mediterranean monk seal hunting in the regional Epipalaeolithic of Southern Iberia. A study of the Nerja Cave site (Málaga, Spain). Quat. Int. 2019, 515, 80–91. [Google Scholar] [CrossRef]
- Androukaki, E.; Adamantopoulou, S.; Dendrinos, P.; Tounta, E.; Kotomatas, S. Causes of mortality in the Mediterranean monk seal (Monachus monachus) in Greece. Contrib. Zool. Ecol. East. Mediterr. Reg. 1999, 1, 405–411. [Google Scholar]
- Karamanlidis, A.A.; Androukaki, E.; Adamantopoulou, S.; Chatzispyrou, A.; Johnson, W.M.; Kotomatas, S.; Papadopoulos, A.; Paravas, V.; Paximadis, G.; Pires, R.; et al. Assessing accidental entanglement as a threat to the Mediterranean monk seal Monachus monachus. Endanger. Species Res. 2008, 5, 205–213. [Google Scholar] [CrossRef]
- Osterhaus, A.; Van De Bildt, M.; Vedder, L.; Martina, B.; Niesters, H.; Vos, J.; Van Egmond, H.; Liem, D.; Baumann, R.; Androukaki, E.; et al. Monk seal mortality: Virus or toxin? Vaccine 1998, 16, 979–981. [Google Scholar] [CrossRef]
- Reyero, M.; Cacho, E.; Martínez, A.; Vázquez, J.; Marina, A.; Fraga, S.; Franco, D.J.M. Evidence of saxitoxin derivatives as causative agents in the 1997 mass mortality of monk seals in the Cape Blanc peninsula. Nat. Toxins 1999, 7, 311–315. [Google Scholar] [CrossRef]
- Pastor, T.; Garza, J.C.; Allen, P.; Amos, W.; Aguilar, A. Low genetic variability in the highly endangered mediterranean monk seal. J. Hered. 2004, 95, 291–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor, T.; Garza, J.C.; Aguilar, A.; Tounta, E.; Androukaki, E. Genetic diversity and differentiation between the two remaining populations of the critically endangered Mediterranean monk seal. Anim. Conserv. 2007, 10, 461–469. [Google Scholar] [CrossRef]
- Karamanlidis, A.A.; Gaughran, S.; Aguilar, A.; Dendrinos, P.; Huber, D.; Pires, R.; Schultz, J.; Skrbinšek, T.; Amato, G. Shaping species conservation strategies using mtDNA analysis: The case of the elusive Mediterranean monk seal (Monachus monachus). Biol. Conserv. 2016, 193, 71–79. [Google Scholar] [CrossRef]
- Gaubert, P.; Justy, F.; Mo, G.; Aguilar, A.; Danyer, E.; Borrell, A.; Dendrinos, P.; Öztürk, B.; Improta, R.; Tonay, A.M.; et al. Insights from 180 years of mitochondrial variability in the endangered Mediterranean monk seal (Monachus monachus). Mar. Mamm. Sci. 2019, 35, 1489–1511. [Google Scholar] [CrossRef]
- Bundone, L.; Panou, A.; Molinaroli, E. On sightings of (vagrant?) monk seals, Monachus monachus, in the Mediterranean Basin and their importance for the conservation of the species. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 554–563. [Google Scholar] [CrossRef]
- Cassoli, P.F.; Fiore, I.; Tagliacozzo, A. Butchery and exploitation of large mammals in the Epigravettian levels of Grotta Romanelli. Anthropozoologica 1997, 25, 309–318. [Google Scholar]
- Di Turo, P. Presenza della foca monaca (Monachus monachus) nell’area mediterranea con particolare riferimento alla Puglia. Thalassia Salentina 1984, 14, 66–84. [Google Scholar] [CrossRef]
- Mo, G. Mediterranean monk seal (Monachus monachus) sightings in Italy (1998–2010) and implications for conservation. Aquat. Mamm. 2011, 37, 236–240. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Bandelt, H.-J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Panou, A.; Jacobs, J.; Panos, D. The endangered mediterranean monk seal Monachus monachus in the Ionian Sea, Greece. Biol. Conserv. 1993, 64, 129–140. [Google Scholar] [CrossRef]
- Panou, A. Monk seal sightings in the central Ionian Sea. A network of fishermen for the protection of the marine resources. In Proceedings of the European Cetacean Society Annual Conference, Istanbul, Turkey, 28 February 2009; p. 6. [Google Scholar]
- Adamantopoulou, S.; Androukaki, E.; Dendrinos, P.; Kotomatas, S.; Paravas, V.; Psaradellis, M.; Tounta, E.; Karamanlidis, A.A. Movements of Mediterranean monk seals (Monachus monachus) in the Eastern Mediterranean Sea. Aquat. Mamm. 2011, 37, 256–261. [Google Scholar] [CrossRef]
- Bayed, A. Further observations of Mediterranean monk seals on the north Atlantic coast of Morocco. Monachus Guard. 2001, 4, 45–47. [Google Scholar]
- Alfaghi, I.A.; Abed, A.S.; Dendrinos, P.; Psaradellis, M.; Karamanlidis, A.A. First confirmed sighting of the mediterranean monk seal (Monachus monachus) in Libya since 1972. Aquat. Mamm. 2013, 39, 81–84. [Google Scholar] [CrossRef]
- Mo, G.; Zotti, A.; Agnesi, S.; Finola, M.G.; Bernardini, D.; Cozzi, B. Age classes and sex differences in the skull of the Mediterranean monk seal, Monachus monachus (Hermann, 1779). A study based on bone shape and density. Anat. Rec. 2009, 292, 544–556. [Google Scholar] [CrossRef]
- Samaranch, R.; González, L.M. Changes in morphology with age in Mediterranean monk seals (Monachus monachus). Mar. Mamm. Sci. 2000, 16, 141–157. [Google Scholar] [CrossRef]
- Bundone, L.; Fai, S.; D’Ambrosio, P.; Onorato, R.; Minonne, F.; Molinaroli, E. Coastal habitat availability for the Mediterranean monk seal, Monachus monachus (Hermann, 1779), in Salento: Preliminary results. Biol. Mar. Mediterr. 2014, 21, 253–254. [Google Scholar]
- Aguilar, A.; Cappozzo, L.H.; Gazo, M.; Pastor, T.; Forcada, J.; Grau, E. Lactation and mother-pup behaviour in the Mediterranean monk seal Monachus monachus: An unusual pattern for a phocid. J. Mar. Biol. Assoc. UK 2007, 87, 93–99. [Google Scholar] [CrossRef]
- Kıraç, C.O.; Ok, M. Diet of a Mediterranean monk seal Monachus monachus in a transitional post-weaning phase and its implications for the conservation of the species. Endanger. Species Res. 2019, 39, 315–320. [Google Scholar] [CrossRef]
- SPA/RAC-UNEP/MAP. On the Occurrence of the Mediterranean Monk Seal Monachus monachus (Hermann, 1779) in the Lebanese Waters (Eastern Mediterranean Sea); Badreddine, A., Limam, A., Ben-Nakhla, L., Eds.; SPA/RAC: Tunis, Tunisia, 2020; p. 12. [Google Scholar]



| Diagnostic Sites | |||||||
|---|---|---|---|---|---|---|---|
| Haplotype ID | GenBank Accession No. | 105 | 114 | 118 | 248 | 279 | 508 |
| MM01 | KT935311 | A | A | A | A | C | G |
| MM02 | KT935307 | G | . | . | . | . | . |
| MM03 | KT935310 | . | . | . | . | . | A |
| MMBR | MT524708 | . | . | . | . | . | A |
| MM05 | KT935309 | . | . | G | . | . | . |
| MM06 | MG570470 | . | . | G | . | T | . |
| MM04 | KT935308 | . | . | G | . | . | A |
| MM07 | MG570469 | . | G | G | G | . | A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fioravanti, T.; Splendiani, A.; Righi, T.; Maio, N.; Lo Brutto, S.; Petrella, A.; Caputo Barucchi, V. A Mediterranean Monk Seal Pup on the Apulian Coast (Southern Italy): Sign of an Ongoing Recolonisation? Diversity 2020, 12, 258. https://doi.org/10.3390/d12060258
Fioravanti T, Splendiani A, Righi T, Maio N, Lo Brutto S, Petrella A, Caputo Barucchi V. A Mediterranean Monk Seal Pup on the Apulian Coast (Southern Italy): Sign of an Ongoing Recolonisation? Diversity. 2020; 12(6):258. https://doi.org/10.3390/d12060258
Chicago/Turabian StyleFioravanti, Tatiana, Andrea Splendiani, Tommaso Righi, Nicola Maio, Sabrina Lo Brutto, Antonio Petrella, and Vincenzo Caputo Barucchi. 2020. "A Mediterranean Monk Seal Pup on the Apulian Coast (Southern Italy): Sign of an Ongoing Recolonisation?" Diversity 12, no. 6: 258. https://doi.org/10.3390/d12060258
APA StyleFioravanti, T., Splendiani, A., Righi, T., Maio, N., Lo Brutto, S., Petrella, A., & Caputo Barucchi, V. (2020). A Mediterranean Monk Seal Pup on the Apulian Coast (Southern Italy): Sign of an Ongoing Recolonisation? Diversity, 12(6), 258. https://doi.org/10.3390/d12060258