Rotating Arrays of Orchid Flowers: A Simple and Effective Method for Studying Pollination in Food Deceptive Plants
Abstract
:1. Introduction
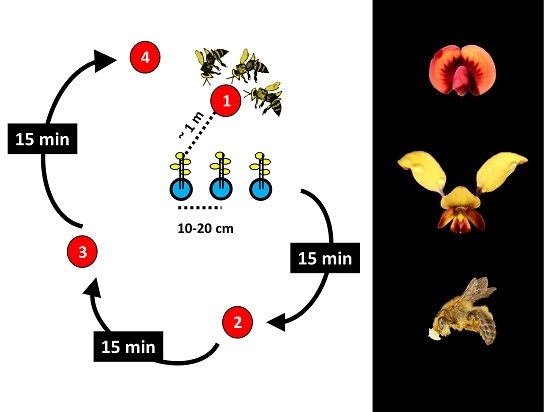
2. Materials and Methods
3. Results
4. Discussion
Recommendations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Renner, S.S. Rewardless Flowers in the Angiosperms and the Role of Insect Cognition in Their Evolution. Plant-Pollinator Interactions: From specialization to Generalization; University of Chicago Press: Chicago, IL, USA, 2007; pp. 123–244. [Google Scholar]
- Ackerman, J. Mechanisms and evolution of food-deceptive pollination systems in orchids. Lindleyana 1986, 1, 108–113. [Google Scholar]
- Nilsson, L.A. Deep flowers for long tongues. Trends Ecol. Evol. 1998, 13, 259–260. [Google Scholar] [CrossRef]
- Jersáková, J.; Johnson, S.D.; Kindlmann, P. Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. Camb. Philos. Soc. 2006, 81, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Kunze, J.; Gumbert, A. The combined effect of color and odor on flower choice behavior of bumble bees in flower mimicry systems. Behav. Ecol. 2001, 12, 447–456. [Google Scholar] [CrossRef]
- Galizia, C.G.; Kunze, J.; Gumbert, A.; Borg-Karlson, A.K.; Sachse, S.; Markl, C.; Menzel, R. Relationship of visual and olfactory signal parameters in a food-deceptive flower mimicry system. Behav. Ecol. 2005, 16, 159–168. [Google Scholar] [CrossRef]
- Jersáková, J.; Jürgens, A.; Šmilauer, P.; Johnson, S.D. The evolution of floral mimicry: Identifying traits that visually attract pollinators. Funct. Ecol. 2012, 26, 1381–1389. [Google Scholar] [CrossRef]
- Van der Cingel, N.A. An Atlas of Orchid Pollination; Balkema Publishers: Rotterdam, The Netherlands, 2001. [Google Scholar]
- Gill, D.E. Fruiting failure, pollination inefficiency, and speciation in orchids. In Speciation and Its Consequences; Otte, D., Endler, J.A., Eds.; Academy of Natural Sciences Publications: Philadelphia, PA, USA, 1989; pp. 458–481. [Google Scholar]
- Neiland, M.R.M.; Wilcock, C.C. Fruit set, nectar reward, and rarity in the Orchidaceae. Am. J. Bot. 1998, 85, 1657–1671. [Google Scholar] [CrossRef]
- Tremblay, R.L.; Ackerman, J.D.; Zimmerman, J.K.; Calvo, R.N. Variation in sexual reproduction in orchids and its evolutionary consequences: A spasmodic journey to diversification. Biol. J. Linn. Soc. 2005, 84, 1–54. [Google Scholar] [CrossRef]
- Scopece, G.; Juillet, N.; MÜller, A.; Schiestl, F.P.; Cozzolino, S. Pollinator attraction in Anacamptis papilionacea (Orchidaceae): A food or a sex promise? Plant Species Biol. 2009, 24, 109–114. [Google Scholar] [CrossRef]
- Brundrett, M.C. A comprehensive study of Orchid seed production relative to pollination traits, plant density and climate in an urban reserve in Western Australia. Diversity 2019, 11, 123. [Google Scholar] [CrossRef] [Green Version]
- Gumbert, A. Color choices by bumble bees (Bombus terrestris): Innate preferences and generalization after learning. Behav. Ecol. Sociobiol. 2000, 48, 36–43. [Google Scholar] [CrossRef]
- Internicola, A.I.; Juillet, N.; Smithson, A.; Gigord, L.D.B. Experimental investigation of the effect of spatial aggregation on reproductive success in a rewardless orchid. Oecologia 2006, 150, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Stoutamire, W.P. Australian terrestrial orchids, thynnid wasps, and pseudocopulation. Am. Orchid Soc. Bull. 1974, 4, 13–18. [Google Scholar]
- Peakall, R. Responses of male Zaspilothynnus trilobatus to females and the orchid it pollinates. Funct. Ecol. 1990, 4, 159–167. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Ayasse, M.; Paulus, H.F.; Lofstedt, C.; Hansson, B.S.; Ibarra, F.; Francke, W. Orchid pollination by sexual swindle. Nature 1999, 399, 421–422. [Google Scholar] [CrossRef]
- Ayasse, M.; Schiestl, F.P.; Paulus, H.F.; Ibarra, F.; Francke, W. Pollinator attraction in a sexually deceptive orchid by means of unconventional chemicals. Proc. R. Soc. B Biol. Sci. 2003, 270, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Gaskett, A.C.; Winnick, C.G.; Herberstein, M.E. Orchid sexual deceit provokes ejaculation. Am. Nat. 2008, 171, E206–E212. [Google Scholar] [CrossRef] [Green Version]
- Phillips, R.D.; Scaccabarozzi, D.; Retter, B.A.; Hayes, C.; Brown, G.R.; Dixon, K.W.; Peakall, R. Caught in the act: Pollination of sexually deceptive trap-flowers by fungus gnats in Pterostylis (Orchidaceae). Ann. Bot. 2014, 113, 629–641. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, M.R.; Peakall, R. Short-term but not long-term patch avoidance in an orchid-pollinating solitary wasp. Behav. Ecol. 2013, 24, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Cuervo, M.; Rakosy, D.; Martel, C.; Schulz, S.; Ayasse, M. Sexual Deception in the Eucera-Pollinated Ophrys leochroma: A Chemical Intermediate between Wasp- and Andrena-Pollinated Species. J. Chem. Ecol. 2017, 43, 469–479. [Google Scholar] [CrossRef]
- Thomson, J.D. Effects of variation in inflorescence size and floral rewards on the visitation rates of traplining pollinators of Aralia hispida. Evol. Ecol. 1988, 2, 65–76. [Google Scholar] [CrossRef]
- Johnson, S.D.; Nilsson, L.A. Pollen carryover, geitonogamy, and the evolution of deceptive pollination systems in orchids. Ecology 1999, 80, 2607–2619. [Google Scholar] [CrossRef]
- Johnson, S.D. Batesian mimicry in the non-rewarding orchid Disa pulchra, and its consequences for pollinator behaviour. Biol. J. Linn. Soc. 2000, 71, 119–132. [Google Scholar] [CrossRef]
- Johnson, S.D.; Peter, C.I.; Nilsson, L.A.; Ågren, J. Pollination success in a deceptive orchid is enhanced by co-occurring rewarding magnet plants. Ecology 2003, 84, 2919–2927. [Google Scholar] [CrossRef] [Green Version]
- Scaccabarozzi, D.; Cozzolino, S.; Guzzetti, L.; Galimberti, A.; Milne, L.; Dixon, K.W.; Phillips, R.D. Masquerading as pea plants: Behavioural and morphological evidence for mimicry of multiple models in an Australian orchid. Ann. Bot. 2018, 122, 1061–1073. [Google Scholar] [CrossRef] [Green Version]
- Western Australian Herbarium. FloraBase—The Western Australian Flora. Department of Biodiversity, Conservation and Attractions. 1998. Available online: https://florabase.dpaw.wa.gov.au/ (accessed on 12 May 2020).
- Dixon, K.W.; Buirchell, B.J.; Collins, M.T. Orchids of Western Australia: Cultivation and Natural History, 2nd ed.; Western Australian Native Orchid Study and Conservation Group: Victoria Park, Perth, Australia, 1989. [Google Scholar]
- Scaccabarozzi, D.; Dixon, K.W.; Tomlinson, S.; Milne, L.; Bohman, B.; Phillips, R.D.; Cozzolino, S. Pronounced differences in visitation by potential pollinators to co-occurring species of Fabaceae in the Southwest Australian biodiversity hotspot. Bot. J. Linn. Soc. in press. [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenALEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [Green Version]
- Bower, C.C. Specific pollinators reveal a cryptic taxon in the bird orchid, Chiloglottis valida sensu lato (Orchidaceae) in south-eastern Australia. Aust. J. Bot. 2006, 54, 53–64. [Google Scholar] [CrossRef]
- Wong, B.B.; Salzmann, C.; Schiestl, F.P. Pollinator attractiveness increases with distance from flowering orchids. Proc. R. Soc. B 2004, 271, S212–S214. [Google Scholar] [CrossRef] [Green Version]
- Batra, S.W. Solitary bees. Sci. Am. 1984, 250, 120–127. [Google Scholar] [CrossRef]
- Johnson, S.D.; Schiestl, F.P. Floral mimicry; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Dyer, F.C. Spatial memory and navigation by honeybees on the scale of the foraging range. J. Exp. Biol. 1996, 199, 147–154. [Google Scholar] [PubMed]
- Goulson, D.; Hawson, S.A.; Stout, J.C. Foraging bumblebees avoid owers already visited by conspecifics or by other bumblebee species. Anim. Behav. 1998, 55, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, B.; Johnson, S.D. The effects of floral mimics and models on each others’ fitness. Proc. R. Soc. B 2006, 273, 969–974. [Google Scholar] [CrossRef] [Green Version]
- Scaccabarozzi, D.; Guzzetti, L.; Phillips, R.D.; Milne, L.; Tommasi, N.; Cozzolino, S.; Dixon, K.D. Ecological factors affecting pollination success in an orchid that mimics multiple species of pea plants (Faboideae). Bot. J. Linn. Soc. submitted. [CrossRef]
- Reiter, N.; Bohman, B.; Flematti, G.R.; Phillips, R.D. Pollination by nectar-foraging thynnine wasps: Evidence of a new specialized pollination system for Australian orchids. Bot. J. Linn. Soc. 2018, 188, 327–337. [Google Scholar] [CrossRef]
- Reiter, N.; Bohman, B.; Batley, M.; Phillips, R.D. Pollination of an endangered Caladenia species (Orchidaceae) by nectar-foraging behaviour of a widespread species of colletid bee. Bot. J. Linn. Soc. 2019, 189, 83–98. [Google Scholar] [CrossRef]
- Reiter, N.; Bohman, B.; Freestone, M.; Brown, G.R.; Phillips, R.D. Pollination by nectar-foraging thynnine wasps in the endangered Caladenia arenaria and Caladenia concolor (Orchidaceae). Aust. J. Bot. 2019, 67, 490–500. [Google Scholar] [CrossRef]
- Phillips, R.D.; Bohman, B.; Brown, G.R.; Tomlinson, S.; Peakall, R. A specialised pollination system using nectar-seeking thynnine wasps in Caladenia nobilis (Orchidaceae). Plant Biol. 2020, 22, 157–166. [Google Scholar] [CrossRef]
- Kunin, W.E. Sex and the single mustard: Population density and pollinator behavior effects on seed-set. Ecology 1993, 74, 2145–2160. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Ayasse, M.; Paulus, H.F.; Erdmann, D.; Francke, W. Variation of floral scent emission and postpollination changes in individual flowers of Ophrys sphegodes subsp. sphegodes. J. Chem. Ecol. 1997, 12, 2881–2895. [Google Scholar] [CrossRef]




| Methods for Orchid Pollination Studies | Description | First Application | Study Orchid Species | Orchid Fruit Set (Average) | Aim | Pollination Strategy | Limitations |
|---|---|---|---|---|---|---|---|
| Baiting Stations | picked inflorescences or potted plants presented randomly in the landscape; from 2 to 15 min trials | SD: Stoutamire, 1974; Peakall, 1990 GFDF: Reiter et al., 2018; 2019 | Drakaea glyptodon Caladenia versicolor Caladenia concolor | 20% 50% 30.5% | attract pollinators | SD GFDF | SD: absence of males influences the effectiveness GFD: proximity to nest sites influences the effectiveness |
| Choice Experiment or Bee Interview Technique | a bifurcated stick presenting two inflorescences (one of the mimic species and another one of the model species, or two orchid inflorescences depending on the strategy) | Thomson, 1988; Johnson & Nilsson, 1999; Johnson, 2000 | Disa pulchra Orchis morio Platanthera chlorantha | 15% 51% 29% | test for food mimicry or test for nectar effect on visitation rate | BFM GFD NR | when the pollination success is low, sufficient replicas are not warranted; presence of nest sites influences the effectiveness |
| Rotating Arrays | systematic rotation of arrays of orchid flowers (picked inflorescences) relative to the position of various model plants; 15 min trials | Scaccabarozzi et al., 2018; 2020 | Diuris brumalis, Diuris magnifica | 3% | increase the visitation rate by insects | BFM | presence and abundance of rewarding model plants determines the effectiveness |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scaccabarozzi, D.; Galimberti, A.; Dixon, K.W.; Cozzolino, S. Rotating Arrays of Orchid Flowers: A Simple and Effective Method for Studying Pollination in Food Deceptive Plants. Diversity 2020, 12, 286. https://doi.org/10.3390/d12080286
Scaccabarozzi D, Galimberti A, Dixon KW, Cozzolino S. Rotating Arrays of Orchid Flowers: A Simple and Effective Method for Studying Pollination in Food Deceptive Plants. Diversity. 2020; 12(8):286. https://doi.org/10.3390/d12080286
Chicago/Turabian StyleScaccabarozzi, Daniela, Andrea Galimberti, Kingsley W. Dixon, and Salvatore Cozzolino. 2020. "Rotating Arrays of Orchid Flowers: A Simple and Effective Method for Studying Pollination in Food Deceptive Plants" Diversity 12, no. 8: 286. https://doi.org/10.3390/d12080286
APA StyleScaccabarozzi, D., Galimberti, A., Dixon, K. W., & Cozzolino, S. (2020). Rotating Arrays of Orchid Flowers: A Simple and Effective Method for Studying Pollination in Food Deceptive Plants. Diversity, 12(8), 286. https://doi.org/10.3390/d12080286