Opportunistic or Non-Random Wildlife Crime? Attractiveness Rather Than Abundance in the Wild Leads to Selective Parrot Poaching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Wild Parrot Surveys
2.3. Poached Parrot Surveys
2.4. Parrot Attractiveness
2.5. Statistical Analyses
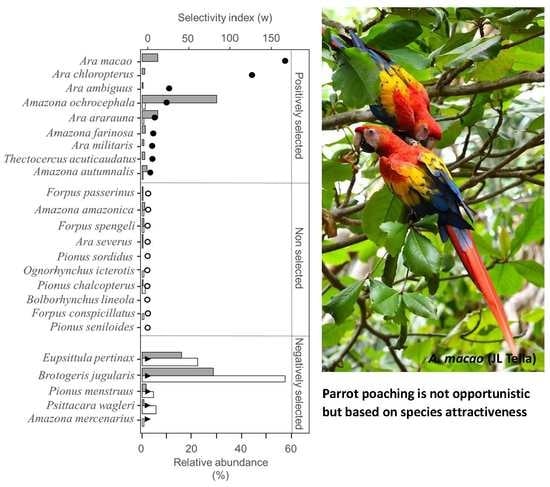
3. Results
4. Discussion
4.1. Parrot Poaching Is Not an Opportunistic, but a Selective Wildlife Crime
4.2. Conservation Implications of Selective Parrot Poaching
4.3. Ecological Implications of Selective Parrot Poaching
4.4. Suggested Conservation Actions
4.5. Further Prospects for Assessing Selective Harvesting
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Young, H.S.; McCauley, D.J.; Galetti, M.; Dirzo, R. Patterns, Causes, and Consequences of Anthropocene Defaunation. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 333–358. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E. Biodiversity: The ravages of guns, nets and bulldozers. Nature 2016, 536, 143–145. [Google Scholar] [CrossRef]
- IPBES. Intergovernment Platform on Biodiversity and Ecosystem Services. Summary for Policymakers of the Global Assessment Report. 2019. Available online: https://www.ipbes.net/sites/default/files (accessed on 12 June 2020).
- Hinsley, A.; Verissimo, D.; Roberts, D.L. Heterogeneity in consumer preferences for orchids in international trade and the potential for the use of market research methods to study demand for wildlife. Biol. Conserv. 2015, 190, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Cassey, P.; Vall-Llosera, M.; Blackburn, T.M. Going cheap: Determinants of bird price in the Taiwanese pet market. PLoS ONE 2015, 10, e0127482. [Google Scholar] [CrossRef] [Green Version]
- Silva, T. Psittaculture: A Manual for the Care and Breeding of Parrots; Nová Exota, Czech Republic & AAL Pty. Ltd.: Bellingen, NSW, Australia, 2018; p. 597. [Google Scholar]
- Bush, E.R.; Baker, S.E.; Macdonald, D.W. Global trade in exotic pets 2006–2012. Conserv. Biol. 2014, 28, 663–676. [Google Scholar] [CrossRef]
- Pires, S.; Clarke, R.V. Are Parrots CRAVED? An Analysis of Parrot Poaching in Mexico. J. Res. Crime Delinq. 2012, 49, 122–146. [Google Scholar] [CrossRef]
- Cantú, J.C.; Sánchez, M.E.; Grosselet, M.; Silve, J. The Illegal Parrot Trade in Mexico: A Comprehensive Assessment; Defenders of Wildlife: Washington, DC, USA, 2007; p. 121. [Google Scholar]
- Pires, S.F. A CRAVED analysis of multiple illicit parrot markets in Peru and Bolivia. Eur. J. Crim. Pol. Res. 2015, 21, 321–336. [Google Scholar] [CrossRef]
- Tella, J.L.; Hiraldo, F. Illegal and legal parrot trade shows a long-term, cross-cultural preference for the most attractive species increasing their risk of extinction. PLoS ONE 2014, 9, e107546. [Google Scholar] [CrossRef] [Green Version]
- Esmail, N.; Wintle, B.C.; Sas-Rolfes, M.; Athanas, A.; Beale, C.M.; Bending, Z.; Dai, R.; Fabinyi, M.; Gluszek, S.; Haenlein, C.; et al. Emerging illegal wildlife trade issues: A global horizon scan. Conserv. Lett. 2020, e12715. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, N.J.M.; García, O.R. Comercio de fauna silvestre en Colombia. Rev. Fac. Nacio. Agron. Medellín 2008, 61, 4618–4645. [Google Scholar]
- DANE. Available online: http://www.dane.gov.co (accessed on 12 June 2020).
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cuervo, A.M.; Aide, T.M.; Clark, M.L.; Etter, A. Land cover change in Colombia: Surprising forest recovery trends between 2001 and 2010. PLoS ONE 2012, 7, e43943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Mahecha, J.V.; Hernández-Camacho, J.I. Loros de Colombia; Conservation International: Bogotá D.C., Colombia, 2002. [Google Scholar]
- Tella, J.L.; Rojas, A.; Carrete, M.; Hiraldo, F. Simple assessments of age and spatial population structure can aid conservation of poorly known species. Biol. Conserv. 2013, 167, 425–434. [Google Scholar] [CrossRef]
- Blanco, G.; Hiraldo, F.; Rojas, A.; Dénes, F.V.; Tella, J.L. Parrots as key multilinkers in ecosystem structure and functioning. Ecol. Evol. 2015, 5, 4141–4160. [Google Scholar] [CrossRef] [Green Version]
- Tella, J.L.; Dénes, F.V.; Zulian, V.; Prestes, N.P.; Martínez, J.; Blanco, G.; Hiraldo, F. Endangered plant-parrot mutualisms: Seed tolerance to predation makes parrots pervasive dispersers of the Parana pine. Sci. Rep. 2016, 6, 31709. [Google Scholar] [CrossRef]
- Baños-Villalba, A.; Blanco, G.; Díaz-Luque, J.A.; Dénes, F.V.; Hiraldo, F.; Tella, J.L. Seed dispersal by macaws shapes the landscape of an Amazonian ecosystem. Sci. Rep. 2017, 7, 7373. [Google Scholar] [CrossRef] [Green Version]
- Dénes, F.V.; Tella, J.L.; Beissinger, S.R. Revisiting methods for estimating parrot abundance and population size. Emu 2018, 118, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Luna, Á.; Romero-Vidal, P.; Hiraldo, F.; Tella, J.L. Cities may save some threatened species but not their ecological functions. PeerJ 2018, 6, e4908. [Google Scholar] [CrossRef]
- Blanco, G.; Bravo, C.; Chamorro, D.; Lovas-Kiss, A.; Hiraldo, F.; Tella, J.L. Herb endozoochory by cockatoos: Is “foliage the fruit20”? Aust. Ecol. 2020, 45, 122–126. [Google Scholar] [CrossRef]
- Wirminghaus, J.O.; Downs, C.T.; Perrin, M.R.; Symes, C.T. Abundance and activity patterns of the Cape parrot (Poicephalus robustus) in two afromontane forests in South Africa. Afr. Zool. 2001, 36, 71–77. [Google Scholar] [CrossRef]
- Salinas-Melgoza, A.; Renton, K. Seasonal variation in activity patterns of juvenile Lilac-crowned parrots in tropical dry forest. Wilson Bull. 2006, 117, 291–295. [Google Scholar] [CrossRef]
- Tella, J.L.; Hernández-Brito, D.; Blanco, G.; Hiraldo, F. Urban sprawl, food subsidies and power lines: An ecological trap for large frugivorous bats in Sri Lanka? Diversity 2020, 12, 94. [Google Scholar] [CrossRef] [Green Version]
- Young, J.C.; Rose, D.C.; Mumby, H.S.; Benitez-Capistros, F.; Derrick, C.J.; Finch, T.; Garcia, C.; Home, C.; Marwaha, E.; Morgans, C.; et al. A methodological guide to using and reporting on interviews in conservation science research. Methods Ecol. Evol. 2018, 9, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.; Buckland, S.T.; Rexstad, E.A.; Laake, J.L.; Strindberg, S.; Hedley, S.L.; Bishop, J.R.; Marques, T.A.; Burnham, K.P. Distance software: Design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 2010, 47, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forshaw, J.M. Parrots of the World: Helm Field Guides; A & C Black Publishers Ltd.: London, UK, 2010. [Google Scholar]
- Manly, B.; McDonald, L.; Thomas, D.; McDonald, T. Resource Selection by Animals: Statistical Design and Analysis for Field Studies, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Rebolo-Ifrán, N.; Tella, J.L.; Carrete, M. Urban conservation hotspots: Predation release allows the grassland-specialist burrowing owl to perform better in the city. Sci. Rep. 2017, 7, 3527. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing (R-3.6.1); R Foundation for StatisticalComputing: Vienna, Austria, 2019. [Google Scholar]
- Sodhi, N.S.; Koh, L.P.; Brook, B.W.; Ng, P.K.L. Southeast Asian biodiversity: An impending disaster. Trends Ecol. Evol. 2004, 19, 654–660. [Google Scholar] [CrossRef]
- Ortiz-von Halle, B. Bird’s-Eye View: Lessons from 50 Years of Bird Trade Regulation; TRAFFIC: Cambridge, UK, 2018. [Google Scholar]
- Tittensor, D.P.; Harfoot, M.; McLardy, C.; Britten, G.L.; Kecse-Nagy, K.; Landry, B.; Outhwaite, W.; Price, B.; Sinovas, P.; Blanc, J.; et al. Evaluating the relationships between the legal and illegal international wildlife trades. Conserv. Lett. 2020, e12724. [Google Scholar] [CrossRef]
- Frynta, D.; Lišková, S.; Bültmann, S.; Burda, H. Being attractive brings advantages: The case of parrot species in captivity. PLoS ONE 2010, 5, e12568. [Google Scholar] [CrossRef]
- Wright, T.F.; Toft, C.A.; Enkerlin-Hoeflich, E.; Gonzalez-Elizondo, J.; Albornoz, M.; Rodríguez-Ferraro, A.; Rojas-Suárez, F.; Sanz, V.; Trujillo, A.; Beissinger, S.R.; et al. Nest Poaching in Neotropical Parrots. Conserv. Biol. 2001, 15, 710–720. [Google Scholar] [CrossRef]
- Herrera, M.; Hennessey, B. Quantifying the illegal parrot trade in Santa Cruz de la Sierra, Bolivia, with emphasis on threatened species. Bird Conserv. Int. 2007, 17, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Vall-llosera, M.; Cassey, P. Physical attractiveness, constraints to the trade and handling requirements drive the variation in species availability in the Australian cagebird trade. Ecol. Econ. 2017, 131, 407–413. [Google Scholar] [CrossRef]
- Beissinger, S.R. Trade in live wild birds: Potentials, Principles and Practices of Sustainable Use. In Conservation of Exploited Species; Reynolds, J.D., Mace, G.M., Redford, K.H., Robinson, J.G., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 182–202. [Google Scholar]
- Cardador, L.; Lattuada, M.; Strubbe, D.; Tella, J.L.; Reino, L.; Figueira, R.; Carrete, M. Regional bans on wild-bird trade modify invasion risks at a global scale. Conserv. Lett. 2017, 10, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Marsden, S.J.; Royle, K. Abundance and abundance change in the world’s parrots. Ibis 2015, 157, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Beissinger, S.R.; Bucher, E.H. Can parrots be conserved through sustainable harvesting? BioScience 1992, 42, 164–173. [Google Scholar] [CrossRef]
- Valle, S.; Collar, N.J.; Harris, W.E.; Marsden, S.J. Trapping method and quota observance are pivotal to population stability in a harvested parrot. Biol. Conserv. 2018, 217, 428–436. [Google Scholar] [CrossRef]
- Olah, G.; Butchart, S.H.M.; Symes, A.; Guzmán, I.M.; Cunningham, R.; Brightsmith, D.J.; Heinsohn, R. Ecological and socio-economic factors affecting extinction risk in parrots. Biodivers. Conserv. 2016, 25, 205–223. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. Version 2019-2. Available online: http://www.iucnredlist.org (accessed on 18 July 2019).
- Martin, R.O. The wild bird trade and African parrots: Past, present and future challenges. Ostrich 2018, 89, 139–143. [Google Scholar] [CrossRef]
- Cardador, L.; Tella, J.L.; Anadón, J.D.; Abellán, P.; Carrete, M. The European trade ban on wild birds reduced invasion risks. Conserv. Lett. 2019, 12, e12631. [Google Scholar] [CrossRef]
- Ribeiro, J.; Reino, L.; Schindler, S.; Strubbe, D.; Vall-llosera, M.; Bastos Araújo, M.; Capinha, C.; Carrete, M.; Mazzoni, S.; Monteiro, M.; et al. Trends in legal and illegal trade of wild birds: A global assessment based on expert knowledge. Biodiv. Conserv. 2019, 28, 3343–3369. [Google Scholar] [CrossRef]
- Martin, R.O.; Senni, C.; D’cruze, N.; Bruschi, N. Tricks of the trade—Legal trade used to conceal Endangered African grey parrots on commercial flights. Oryx 2019, 53, 213. [Google Scholar] [CrossRef] [Green Version]
- Reuter, K.E.; Rodriguez, L.; Hanitriniaina, S.; Schaefer, M.S. Ownership of parrots in Madagascar: Extent and conservation implications. Oryx 2019, 53, 582–588. [Google Scholar] [CrossRef]
- Regueira, R.F.S.; Bernard, E. Wildlife sinks: Quantifying the impact of illegal bird trade in street markets in Brazil. Biol. Conserv. 2012, 149, 16–22. [Google Scholar] [CrossRef]
- Alves, R.R.N.; Lima, J.R.D.F.; Araujo, H.F.P. The live bird trade in Brazil and its conservation implications: An overview. Bird Conserv. Int. 2013, 23, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Daut, E.F.; Brightsmith, D.J.; Mendoza, A.P.; Puhakka, L.; Peterson, M.J. Illegal domestic bird trade and the role of export quotas in Peru. J. Nat. Conserv. 2015, 27, 44–53. [Google Scholar] [CrossRef]
- Biddle, R.; Ponce, I.S.; Cun, P.; Tollington, S.; Jones, M.; Marsden, S.; Devenish, C.; Horstman, E.; Berg, K.; Pilgrim, M. Conservation status of the recently described Ecuadorian Amazon parrot Amazona lilacina. Bird Conserv. Int. 2020, in press. [Google Scholar] [CrossRef]
- Sánchez-Mercado, A.; Asmüssen, M.; Rodríguez, J.P.; Moran, L.; Cardozo-Urdaneta, A.; Morales, L.I. Illegal trade of the Psittacidae in Venezuela. Oryx 2020, 54, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.B.C.; Green, J.M.; Prawiradilaga, D.M.; Giam, X.; Hikmatullah, D.; Putra, C.A.; Wilcove, D.S. Using market data and expert opinion to identify overexploited species in the wild bird trade. Biol. Conserv. 2015, 187, 51–60. [Google Scholar] [CrossRef]
- Berkunsky, I.; Quillfeldt, P.; Brightsmith, D.J.; Abbud, M.C.; Aguilar, J.M.R.E.; Alemán-Zelaya, U.; Aramburú, R.M.; Arce Arias, A.; Balas McNab, R.; Balsby, T.J.S.; et al. Current threats faced by Neotropical parrot populations. Biol. Conserv. 2017, 214, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Pires, S.F.; Moreto, W.D. Preventing Wildlife Crimes: Solutions That Can Overcome the “Tragedy of the Commons”. Eur. J. Crim. Policy Res. 2011, 17, 101–123. [Google Scholar] [CrossRef]
- Robinson, J.G.; Bennett, E.L. Having your wildlife and eating it too: An analysis of hunting sustainability across tropical ecosystems. Anim. Conserv. 2004, 7, 397–408. [Google Scholar] [CrossRef]
- Fryxell, J.M.; Packer, C.; McCann, K.; Solberg, E.J.; Sæther, B.E. Resource management cycles and the sustainability of harvested wildlife populations. Science 2010, 328, 903–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Mercado, A.; Cardozo-Urdaneta, A.; Moran, L.; Ovalle, L.; Arvelo, M.Á.; Morales-Campos, J.; Coyle, B.; Braun, M.J.; Rodríguez-Clark, K.M. Social network analysis reveals specialized trade in an Endangered songbird. Anim. Conserv. 2020, 23, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Pires, S.; Herrera, M.; Tella, J.L. Spatial, temporal and age sources of variation in parrot poaching in Bolivia. Bird Conserv. Int. 2018, 26, 293–306. [Google Scholar] [CrossRef]
- Carrascal, J.; Chacón, J.; Ochoa, V. Ingreso de psittacidos al centro de atención de fauna (CAV–CVS), durante los años 2007–2009. Rev. MVZ Córdoba 2013, 18, 3414–3419. [Google Scholar] [CrossRef] [Green Version]
- Young, A.M.; Hobson, E.A.; Lackey, L.B.; Wright, T.F. Survival on the ark: Life-history trends in captive parrots. Anim. Conserv. 2012, 15, 28–43. [Google Scholar] [CrossRef] [Green Version]
- Toft, C.A.; Wright, T.F. Parrots of the Wild: A Natural History of the World’s Most Captivating Birds; University of California Press: Berkeley, CA, USA, 2015. [Google Scholar]
- Blanco, G.; Hiraldo, G.; Tella, J.L. Ecological functions of parrots: An integrative perspective from plant life cycle to ecosystem functioning. Emu 2018, 118, 36–49. [Google Scholar] [CrossRef]
- Montesinos-Navarro, A.; Hiraldo, F.; Tella, J.L.; Blanco, G. Network structure embracing mutualism-antagonism continuums increases community robustness. Nat. Ecol. Evol. 2017, 1, 1661. [Google Scholar] [CrossRef]
- Tella, J.L.; Baños-Villalba, A.; Hernández-Brito, D.; Rojas, A.; Pacífico, E.; Díaz-Luque, J.A.; Carrete, M.; Blanco, G.; Hiraldo, F. Parrots as overlooked seed dispersers. Front. Ecol. Environ. 2015, 13, 338–339. [Google Scholar] [CrossRef] [Green Version]
- Tella, J.L.; Blanco, G.; Dénes, F.V.; Hiraldo, F. Overlooked parrot seed dispersal in Australia and South America: Insights on the evolution of dispersal syndromes and seed size in Araucaria trees. Front. Ecol. Evol. 2019, 7, 82. [Google Scholar] [CrossRef] [Green Version]
- Blanco, G.; Tella, J.L.; Díaz-Luque, J.A.; Hiraldo, F. Multiple external seed dispersers challenge the megafaunal syndrome anachronism and the surrogate ecological function of livestock. Front. Ecol. Evol. 2019, 7, 328. [Google Scholar] [CrossRef] [Green Version]
- Tella, J.L.; Hiraldo, F.; Pacífico, E.; Díaz-Luque, J.A.; Dénes, F.V.; Fontoura, F.M.; Guedes, N.; Blanco, G. Conserving the diversity of ecological interactions: The role of two threatened macaw species as legitimate dispersers of “megafaunal” fruits. Diversity 2020, 12, 45. [Google Scholar] [CrossRef] [Green Version]
- Blanco, G.; Bravo, C.; Pacífico, E.; Chamorro, D.; Speziale, K.; Lambertucci, S.; Hiraldo, F.; Tella, J.L. Internal seed dispersal by parrots: An overview of a neglected mutualism. PeerJ 2017, 4, e1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, C.; Chamorro, D.; Hiraldo, F.; Speziale, K.; Lambertucci, S.A.; Tella, J.L.; Blanco, G. Physiological dormancy broken by endozoochory: Austral parakeets (Enicognathus ferrugineus) as legitimate dispersers of Calafate (Berberis microphylla) in the Patagonian Andes. J. Plant Ecol. 2020, in press. [Google Scholar] [CrossRef]
- Hernández-Brito, D.; Romero-Vidal, P.; Hiraldo, F.; Blanco, G.; Díaz-Luque, J.A.; Barbosa, J.M.; Symes, C.T.; White, T.K.; Pacífico, E.C.; Sebastián-González, E.; et al. Caught on camera: Epizoochory in parrots as an overlooked yet widespread plant-animal mutualism. Ecology 2020. under review. [Google Scholar]
- Sebastián-González, E.; Hiraldo, F.; Blanco, G.; Hernández-Brito, D.; Romero-Vidal, P.; Carrete, M.; Gómez-Llanos, E.; Pacífico, E.; Díaz-Luque, J.A.; Denés, F.V.; et al. The extent, frequency and ecological functions of food wasting by parrots. Sci. Rep. 2019, 9, 15280. [Google Scholar] [CrossRef] [Green Version]
- Moleón, M.; Sánchez-Zapata, J.A.; Donázar, J.A.; Revilla, E.; Martín-López, B.; Gutiérrez-Cánovas, C.; Getz, W.M.; Morales-Reyes, Z.; Campos-Arceiz, A.; Crowder, L.B.; et al. Rethinking megafauna. Proc. Roy. Soc. Lond. B 2020, 287, 20192643. [Google Scholar] [CrossRef]
- Barbosa, A.E.A.; Tella, J.L. How much does it cost to save a species from extinction? Costs and rewards of conserving the Lear’s macaw. Roy. Soc. Open Sci. 2019, 6, 190190. [Google Scholar] [CrossRef] [Green Version]
- Briceño-Linares, J.M.; Rodríguez, J.P.; Rodríguez-Clark, K.M.; Rojas-Suárez, F.; Millán, P.A.; Vittori, E.G.; Carrasco-Muñoz, M. Adapting to changing poaching intensity of yellow-shouldered parrot (Amazona barbadensis) nestlings in Margarita Island, Venezuela. Biol. Conserv. 2011, 144, 1188–1193. [Google Scholar] [CrossRef]
- Brightsmith, D.J.; Stronza, A.; Holle, K. Ecotourism, conservation biology, and volunteer tourism: A mutually beneficial triumvirate. Biol. Conserv. 2008, 141, 2832–2842. [Google Scholar] [CrossRef]
- Reuter, P.; O’Regan, D. Smuggling wildlife in the Americas: Scale, methods, and links to other organised crimes. Glob. Crime 2017, 18, 77–99. [Google Scholar] [CrossRef]
- Holden, M.H.; Biggs, D.; Brink, H.; Bal, P.; Rhodes, J.; McDonald-Madden, E. Increase anti-poaching law-enforcement or reduce demand for wildlife products? A framework to guide strategic conservation investments. Conserv. Lett. 2019, 12, e12618. [Google Scholar] [CrossRef] [Green Version]
- Nijman, V.; Langgeng, A.; Helene, B.; Imron, M.A.; Nekaris, K.A.I. Wildlife trade, captive breeding and the imminent extinction of a songbird. Glob. Ecol. Conserv. 2018, 15, e00425. [Google Scholar] [CrossRef]
- Coetzer, W.G.; Downs, C.T.; Perrin, M.R.; Willows-Munro, S. Testing of microsatellite multiplexes for individual identification of Cape Parrots (Poicephalus robustus): Paternity testing and monitoring trade. PeerJ 2017, 5, e2900. [Google Scholar] [CrossRef] [Green Version]
- Hogg, C.J.; Dennison, S.; Frankham, G.J.; Hinds, M.; Johnson, R.N. Stopping the spin cycle: Genetics and bio-banking as a tool for addressing the laundering of illegally caught wildlife as ‘captive-bred’. Conserv. Gen. Res. 2018, 10, 237–246. [Google Scholar] [CrossRef]
- Carrete, M.; Tella, J.L. Rapid loss of antipredatory behaviour in captive-bred birds is linked to current avian invasions. Sci. Rep. 2015, 5, 18274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abellán, P.; Tella, J.L.; Carrete, M.; Cardador, L.; Anadón, J.D. Climatic matching drives spread rate but not establishment success in recent unintentional bird introductions. Proc. Nat. Acad. Sci. USA 2017, 114, 201704815. [Google Scholar] [CrossRef] [Green Version]
- Botero-Delgadillo, E.; Páez, C.A. Estado actual del conocimiento y conservación de los loros amenazados de Colombia. Conserv. Colomb. 2011, 14, 86–151. [Google Scholar]
- Botero-Delgadillo, E.; Páez, C.A. Plan de acción para la conservación de los loros amenazados de Colombia 2010–2020: Avances, logros y perspectivas. Conserv. Colomb. 2011, 14, 7–16. [Google Scholar]
- Thomas-Walters, L.; Veríssimo, D.; Gadsby, E.; Roberts, D.; Smith, R.J. Taking a more nuanced look at behavior change for demand reduction in the illegal wildlife trade. Conserv. Sci. Pract. 2020, 10, e248. [Google Scholar] [CrossRef]
- Morinha, F.; Carrete, M.; Tella, J.L.; Blanco, G. High prevalence of novel beak and feather disease virus in sympatric invasive parakeets introduced to Spain from Asia and South America. Diversity 2020, 12, 192. [Google Scholar] [CrossRef]
- Harris, R.B.; Wall, W.A.; Allendorf, F.W. Genetic consequences of hunting: What do we know and what should we do? Wildl. Soc. Bull. 2002, 30, 634–643. [Google Scholar]
- Coltman, D.W.; O’Donoghue, P.; Jorgenson, J.T.; Hogg, J.T.; Strobeck, C.; Festa-Bianchet, M. Undesirable evolutionary consequences of trophy hunting. Nature 2003, 426, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, F.W.; England, P.R.; Luikart, G.; Ritchie, P.A.; Ryman, N. Genetic effects of harvest on wild animal populations. Trends Ecol. Evol. 2008, 23, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, F.W.; Hard, J.J. Human-induced evolution caused by unnatural selection through harvest of wild animals. Proc. Nat. Acad. Sci. USA 2009, 106, 9987–9994. [Google Scholar] [CrossRef] [Green Version]
- Mysterud, A. Selective harvesting of large mammals: How often does it result in directional selection? J. Appl. Ecol. 2011, 48, 827–834. [Google Scholar] [CrossRef]
- Segura, L.N.; Perelló, M.; Gress, N.H.; Ontiveros, R. The lack of males due to illegal trapping is causing polygyny in the globally endangered Yellow Cardinal Gubernatrix cristata. Ornithol. Res. 2019, 27, 40–43. [Google Scholar] [CrossRef]
- Corlatti, L.; Sanz-Aguilar, A.; Tavecchia, G.; Gugiatti, A.; Pedrotti, L. Unravelling the sex-and age-specific impact of poaching mortality with multievent modeling. Front. Zool. 2019, 16, 20. [Google Scholar] [CrossRef]
- Heinrich, S.; Ross, J.V.; Gray, T.N.; Delean, S.; Marx, N.; Cassey, P. Plight of the commons: 17 years of wildlife trafficking in Cambodia. Biol. Conserv. 2020, 241, 108379. [Google Scholar] [CrossRef]
- Marshall, H.; Collar, N.J.; Lees, A.C.; Moss, A.; Yuda, P.; Marsden, S.J. Spatio-temporal dynamics of consumer demand driving the Asian Songbird Crisis. Biol. Conserv. 2020, 241, 108237. [Google Scholar] [CrossRef]







| Species | Detections | Total Count | Density (ind/km2) | w (m) | Relative Abundance (ind/km) |
|---|---|---|---|---|---|
| Amazona amazonica | 24 | 93 | 0.2881 | 200 | 0.0422 |
| Amazona autumnalis | 10 | 46 | 0.0307 | 307 | 0.0209 |
| Amazona ochrocephala | 43 | 106 | 0.2214 | 348 | 0.0481 |
| Ara ararauna | 16 | 60 | 0.0570 | 757 | 0.0272 |
| Ara severus | 15 | 30 | 0.0395 | 365 | 0.0136 |
| Brotogeris jugularis | 260 | 1678 | 3.9032 | 420 | 0.7620 |
| Eupsittula pertinax | 168 | 1669 | 2.7928 | 235 | 0.7579 |
| Forpus passerinus | 10 | 15 | 0.1546 | 45 | 0.0068 |
| Pionus chalcopterus | 14 | 115 | 0.0621 | 650 | 0.0522 |
| Pionus menstruus | 76 | 356 | 0.8029 | 397 | 0.1617 |
| Psittacara wagleri | 19 | 322 | 0.9090 | 270 | 0.1462 |
| Species | Size | Body | Head | Color | Speech | Price | N Wild | N Pet | W | |
|---|---|---|---|---|---|---|---|---|---|---|
| Amazona amazonica (Aam) | 31 | 0.2 | 1 | 3 | 1.5 (1–2) | 34.85 (-) | 93 | 12 | 1.17 | |
| Amazona autumnalis (Aau) | 34 | 0.2 | 1 | 3 | 1.5 (1–2) | 61 | 25 | 3.73 | * | |
| Amazona farinosa (Afa) | 38 | 0.2 | 0.2 | 1 | 1.9 (1–2) | 38.72 (13.4) | 20 | 18 | 8.18 | * |
| Amazona mercenarius (Ame) | 34 | 0.2 | 0 | 1 | 1.0 (1–1) | 93 | 0 | 0.00 | * | |
| Amazona ochrocephala (Aoc) | 31 | 0.2 | 0.8 | 2 | 2.0 (1–2) | 34.41 (16.0) | 136 | 359 | 24.00 | * |
| Ara ambiguus (Aab) | 85 | 1.5 | 0.5 | 3 | 1.5 (1–2) | 0 | 3 | 27.28 | * | |
| Ara ararauna (Aar) | 85 | 2 | 1.8 | 3 | 0.9 (0–2) | 43.57 (20.5) | 80 | 76 | 8.64 | * |
| Ara chloropterus (Ach) | 90 | 2 | 2 | 3 | 1.5 (1–2) | 0 | 14 | 127.28 | * | |
| Ara macao (Ama) | 85 | 2 | 2 | 3 | 2.0 (2–2) | 145.22 (0.00) | 4 | 74 | 168.20 | * |
| Ara militaris (Ami) | 75 | 1.5 | 0.5 | 3 | 1.5 (0–2) | 10 | 7 | 6.36 | * | |
| Ara severus (Ase) | 46 | 1 | 0 | 2 | 1.0 (1–1) | 54 | 5 | 0.84 | ||
| Bolborhynchus lineola (Blin) | 16 | 0 | 0 | 1 | 0.0 (0–0) | 1 | 0 | 0.00 | ||
| Brotogeris jugularis (Bju) | 18 | 0 | 0 | 1 | 0.4 (0–2) | 6.53 (1.45) | 6230 | 344 | 0.50 | * |
| Eupsittula pertinax (Epe) | 25 | 0.1 | 0 | 1 | 0.5 (0–2) | 5.68 (3.67) | 2445 | 189 | 0.70 | * |
| Forpus conspicillatus (Fco) | 12 | 0.1 | 0 | 1 | 0.0 (0–0) | 83 | 0 | 0.00 | ||
| Forpus passerinus (Fpa) | 12 | 0.1 | 0 | 1 | 0.0 (0–0) | 5.81 (0.00) | 35 | 6 | 1.56 | |
| Forpus spengeli (Fsp) | 12 | 0.1 | 0 | 1 | 0.0 (0–0) | 80 | 9 | 1.02 | ||
| Hapalopsittaca fuertesi (Hfu) | 23 | 0.5 | 0.9 | 3 | 0.0 (0–0) | 1 | 0 | - | ||
| Ognorhynchus icterotis (Oic) | 42 | 0.8 | 0.8 | 2 | 0.5 (0–1) | 85 | 2 | 0.21 | ||
| Pionus chalcopterus (Pch) | 29 | 0.2 | 0 | 2 | 0.0 (0–0) | 134 | 3 | 0.21 | ||
| Pionus menstruus (Pme) | 28 | 0.3 | 1.5 | 2 | 0.2 (0–2) | 16.46 (9.22) | 497 | 19 | 0.35 | * |
| Pionus seniloides (Pse) | 30 | 0.2 | 0 | 2 | 0.0 (0–0) | 2 | 0 | 0.00 | ||
| Pionus sordidus (Pso) | 28 | 0.2 | 0 | 1 | 0.0 (0–0) | 17 | 1 | 0.52 | ||
| Psittacara wagleri (Pwa) | 36 | 0 | 0.5 | 2 | 0.5 (0–1) | 628 | 10 | 0.14 | * | |
| Pyrilia haematotis (Pha) | 21 | 0.1 | 0 | 2 | 0.0 (0–0) | 0 | 1 | - | ||
| Pyrilia pyrilia (Ppy) | 24 | 0.1 | 2 | 3 | 0.0 (0–0) | 1 | 0 | - | ||
| Thectocercus acuticaudatus (Tac) | 37 | 0 | 0.1 | 1 | 0.5 (0–1) | 20 | 13 | 5.91 | * | |
| Touit batavicus (Tba) | 14 | 0.1 | 0 | 3 | 0.0 (0–0) | 1 | 0 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Vidal, P.; Hiraldo, F.; Rosseto, F.; Blanco, G.; Carrete, M.; Tella, J.L. Opportunistic or Non-Random Wildlife Crime? Attractiveness Rather Than Abundance in the Wild Leads to Selective Parrot Poaching. Diversity 2020, 12, 314. https://doi.org/10.3390/d12080314
Romero-Vidal P, Hiraldo F, Rosseto F, Blanco G, Carrete M, Tella JL. Opportunistic or Non-Random Wildlife Crime? Attractiveness Rather Than Abundance in the Wild Leads to Selective Parrot Poaching. Diversity. 2020; 12(8):314. https://doi.org/10.3390/d12080314
Chicago/Turabian StyleRomero-Vidal, Pedro, Fernando Hiraldo, Federica Rosseto, Guillermo Blanco, Martina Carrete, and José L. Tella. 2020. "Opportunistic or Non-Random Wildlife Crime? Attractiveness Rather Than Abundance in the Wild Leads to Selective Parrot Poaching" Diversity 12, no. 8: 314. https://doi.org/10.3390/d12080314
APA StyleRomero-Vidal, P., Hiraldo, F., Rosseto, F., Blanco, G., Carrete, M., & Tella, J. L. (2020). Opportunistic or Non-Random Wildlife Crime? Attractiveness Rather Than Abundance in the Wild Leads to Selective Parrot Poaching. Diversity, 12(8), 314. https://doi.org/10.3390/d12080314