Lack of Cascading Effects of Eurasian Lynx Predation on Roe Deer to Soil and Plant Nutrients
Abstract
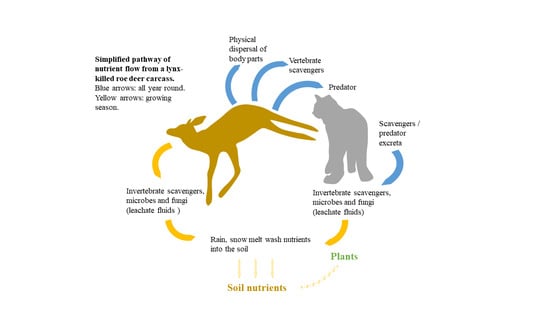
:1. Introduction
- (1)
- Lynx-killed roe deer carcasses will have an effect on nutrient levels in the surrounding soil and vegetation, so that soil and vegetation parameters would be changed to a greater extent closer to the center of the carcass and decline farther away from the carcass [21].
- (2)
2. Materials and Methods
2.1. Study Area
2.2. Study Design
2.3. Laboratory Analyses
2.4. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, D.W.; Peterson, R.O.; Houston, D.B. Yellowstone after wolves. Bioscience 2003, 53, 330–340. [Google Scholar] [CrossRef]
- Berger, J.; Smith, D.W. Restoring functionality in Yellowstone with recovering carnivores: Gains and uncertainties. In Large Carnivores and the Conservation of Biodiversity; Ray, J.C., Redford, K.H., Steneck, R.S., Berger, J., Eds.; Island Press: Washington, DC, USA, 2005; pp. 100–109. [Google Scholar]
- McShea, W.J. Forest ecosystems without carnivores: When ungulates rule the world. In Large Carnivores and the Conservation of Biodiversity; Ray, J.C., Redford, K.H., Steneck, R.S., Berger, J., Eds.; Island Press: Washington, DC, USA, 2005; pp. 138–153. [Google Scholar]
- Ray, J.C. Large carnivorous animals as tools for conserving biodiversity: Assumptions and uncertainties. In Large Carnivores and the Conservation of Biodiversity; Ray, J.C., Redford, K.H., Steneck, R.S., Berger, J., Eds.; Island Press: Washington, DC, USA, 2005; pp. 34–56. [Google Scholar]
- Soule, M.E.; Estes, J.A.; Miller, B.; Honnold, D.L. Strongly interacting species. conservation policy, management, and ethics. Bioscience 2005, 55, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Ray, J.C.; Redford, K.H.; Berger, J.; Steneck, R.S. Conclusion: Is large carnivore conservation equivalent to biodiversity conservation and how can we achieve both? In Large Carnivores and the Conservation of Biodiversity; Ray, J.C., Redford, K.H., Steneck, R.S., Berger, J., Eds.; Island Press: Washington, DC, USA, 2005; pp. 400–428. [Google Scholar]
- Pace, M.L.; Cole, J.J.; Carpenter, S.R.; Kitchell, J.F. Trophic cascades revealed in diverse ecosystems. Trends Ecol. Evol. 1999, 14, 483–488. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Hamback, P.A.; Beckerman, A.P. Trophic cascades in terrestrial systems: A review of the effects of carnivore removals on plants. Am. Nat. 2000, 155, 141–153. [Google Scholar] [CrossRef]
- Hobbs, N.T. Large herbivores as sources of disturbance in ecosystems. In Large Herbivore Ecology, Ecosystem Dynamics and Conservation; Pastor, J., Danell, K., Duncan, P., Bergström, R., Eds.; Cambridge University Press: Cambridge, MA, USA, 2006; pp. 261–288. [Google Scholar]
- Ripple, W.J.; Beschta, R.L. Linking wolves and plants: Aldo Leopold on trophic cascades. Bioscience 2005, 55, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Berger, J.; Stacey, P.B.; Bellis, L.; Johnson, M.P. A mammalian predator-prey imbalance: Grizzly bear and wolf extinction affect avian neotropical migrants. Ecol. Appl. 2001, 11, 947–960. [Google Scholar] [CrossRef]
- DeVault, T.L.; Rhodes, O.E.; Shivik, J.A. Scavenging by vertebrates: Behavioral, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. Oikos 2003, 102, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.S.; Durant, S.M.; Caro, T.M. Patterns of scavenger arrival at cheetah kills in Serengeti National Park Tanzania. Afr. J. Ecol. 2007, 45, 275–281. [Google Scholar] [CrossRef]
- Selva, N.; Fortuna, M.A. The nested structure of a scavenger community. Proc. R. Soc. B Biol. Sci. 2007, 274, 1101–1108. [Google Scholar] [CrossRef] [Green Version]
- Selva, N.; Jedrzejewska, B.; Jedrzejewski, W.; Wajrak, A. Scavenging on European bison carcasses in Bialowieza Primeval Forest (eastern Poland). Ecoscience 2003, 10, 303–311. [Google Scholar] [CrossRef]
- Selva, N.; Jedrzejewska, B.; Jedrzejewski, W.; Wajrak, A. Factors affecting carcass use by a guild of scavengers in European temperate woodland. Can. J. Zool. 2005, 83, 1590–1601. [Google Scholar] [CrossRef]
- Wilmers, C.C.; Crabtree, R.L.; Smith, D.W.; Murphy, K.M.; Getz, W.M. Trophic facilitation by introduced top predators: Grey wolf subsidies to scavengers in Yellowstone National Park. J. Anim. Ecol. 2003, 72, 909–916. [Google Scholar] [CrossRef] [Green Version]
- Bump, J.K.; Peterson, R.O.; Vucetich, J.A. Wolves modulate soil nutrient heterogeneity and foliar nitrogen by configuring the distribution of ungulate carcasses. Ecology 2009, 90, 3159–3167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danell, K.; Berteaux, D.; Brathen, K.A. Effect of muskox carcasses on nitrogen concentration in tundra vegetation. Arctic 2002, 55, 389–392. [Google Scholar] [CrossRef] [Green Version]
- Melis, C.; Selva, N.; Teurlings, I.; Skarpe, C.; Linnell, J.D.C.; Andersen, R. Soil and vegetation nutrient response to bison carcasses in Bialeowieza Primeval Forest, Poland. Ecol. Res. 2007, 22, 807–813. [Google Scholar] [CrossRef]
- Towne, E.G. Prairie vegetation and soil nutrient responses to ungulate carcasses. Oecologia 2000, 122, 232–239. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Hawlena, D.; Trussell, G.C. Predator control of ecosystem nutrient dynamics. Ecol. Lett. 2010, 13, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Naiman, R.J.; Bilby, R.E.; Schindler, D.E.; Helfield, J.M. Pacific salmon, nutrients, and the dynamics of freshwater and riparian ecosystems. Ecosystems 2002, 5, 399–417. [Google Scholar] [CrossRef]
- Putman, R.J. Carrion and Dung: The Decomposition of Animal Waste; Edward Arnold: London, UK, 1983. [Google Scholar]
- Carter, D.O.; Yellowlees, D.; Tibbett, M. Cadaver decomposition in terrestrial ecosystems. Naturwissenschaften 2007, 94, 12–24. [Google Scholar] [CrossRef] [Green Version]
- Barton, P.S.; Cunningham, S.A.; Lindenmayer, D.B.; Manning, A.D. The role of carrion in maintaining biodiversity and ecological processes in terrestrial ecosystems. Oecologia 2013, 171, 761–772. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setala, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Bump, J.K.; Webster, C.R.; Vucetich, J.A.; Peterson, R.O.; Shields, J.M.; Powers, M.D. Ungulate Carcasses Perforate Ecological Filters and Create Biogeochemical Hotspots in Forest Herbaceous Layers Allowing Trees a Competitive Advantage. Ecosystems 2009, 12, 996–1007. [Google Scholar] [CrossRef]
- DeVault, T.L.; Brisbin, I.L.; Rhodes, O.E. Factors influencing the acquisition of rodent carrion by vertebrate scavengers and decomposers. Can. J. Zool. 2004, 82, 502–509. [Google Scholar] [CrossRef]
- Ray, R.R.; Seibold, H.; Heurich, M. Invertebrates outcompete vertebrate facultative scavengers in simulated lynx kills in the Bavarian Forest National Park, Germany. Anim. Biodiv. Conserv. 2014, 37, 77–88. [Google Scholar]
- Turner, K.L.; Abernethy, E.F.; Conner, L.M.; Olin, E.; Beasley, J.C. Abiotic and biotic factors modulate carrion fate and vertebrate scavenging communities. Ecology 2017, 98, 2413–2424. [Google Scholar] [CrossRef]
- Nilsen, E.B.; Linnell, J.D.C.; Odden, J.; Andersen, R. Climate, season, and social status modulate the functional response of an efficient stalking predator: The Eurasian lynx. J. Anim. Ecol. 2009, 78, 741–751. [Google Scholar] [CrossRef]
- Odden, J.; Linnell, J.D.C.; Andersen, R. Diet of Eurasian lynx, Lynx lynx, in the boreal forest of southeastern Norway: The relative importance of livestock and hares at low roe deer density. Eur. J. Wildlife Res. 2006, 52, 237–244. [Google Scholar] [CrossRef]
- Novozamsky, I.; Houba, V.J.G.; Temminghoff, E.; van der Lee, J.J. Determination of ‘total’ N and ‘total’ P in a single soil digest. Neth. J. Agric. Sci. 1984, 32, 322–324. [Google Scholar]
- Walinga, I.; Van Der Lee, J.J.; Houba, V.J.G.; Van Vark, W.; Novozamsky, I. Digestion in tubes with H2SO4-salicylic acid- H2O2 and selenium and determination of Ca, K, Mg, N, Na, P, Zn. In Plant Analysis Manual; Walinga, I., Van Der Lee, J.J., Houba, V.J.G., Van Vark, W., Novozamsky, I., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 7–45. [Google Scholar]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effect Models in S and S-Plus; Springer: New York, NY, USA, 2002. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.r-project.org/ (accessed on 12 September 2020).
- Bartoń, K. MuMIn: Multi-Model Inference; R Package Version 1.43.17; 2020. Available online: https://www.rdocumentation.org/packages/MuMIn/versions/1.43.17 (accessed on 12 September 2020).
- Bates, D. lme4: Linear Mixed-Effects Models Using S4 Classes; 0.99875-6. 2007. Available online: https://cran.r-project.org/web/packages/lme4/index.html (accessed on 12 September 2020).
- Barton, P.S.; McIntyre, S.; Evans, M.J.; Bump, J.K.; Cunningham, S.A.; Manning, A.D. Substantial long-term effects of carcass addition on soil and plants in a grassy eucalypt woodland. Ecosphere 2016, 7, e01537. [Google Scholar] [CrossRef]
- Andersen, R.; Duncan, P.; Linnell, J.D.C. The European Roe Deer: The Biology of Success; Scandinavian University Press: Oslo, Norway, 1998; Volume 376. [Google Scholar]
- Okarma, H.; Jedrzejewski, W.; Schmidt, K.; Kowalczyk, R.; Jedrzejewska, B. Predation of Eurasian lynx on roe deer and red deer in Bialowieza Primeval Forest, Poland. Acta Theriol. 1997, 42, 203–224. [Google Scholar] [CrossRef] [Green Version]
- Breitenmoser, U.; Breitenmoser-Würsten, C. Der Luchs: Ein Grossraubtier in der Kulturlandschaft; Salm Verlag: Bern, Switzerland, 2008. [Google Scholar]
- Krasinska, M.; Krasinski, Z.A. Body mass and measurements of the European bison during postnatal development. Acta Theriol. 2002, 47, 85–106. [Google Scholar] [CrossRef]
- Klimešová, J.; de Bello, F. CLO-PLA: The database of clonal and bud bank traits of Central European flora. J. Veg. Sci. 2009, 20, 511–516. [Google Scholar] [CrossRef]
- Klimešová, J.; Klimeš, L. Clo-Pla3—Database of Clonal Growth of Plants from Central Europe. 2006. Available online: https://clopla.butbn.cas.cz (accessed on 12 September 2020).
- Tolvanen, A. Differences in Recovery between a Deciduous and an Evergreen Ericaceous Clonal Dwarf Shrub after Simulated Aboveground Herbivory and Belowground Damage. Can. J. Bot. 1994, 72, 853–859. [Google Scholar] [CrossRef]
- Shurin, J.B.; Markel, R.W.; Matthews, B. Comparing trophic cascades across ecosystems. In Trophic Cascades: Predators, Prey and the Changing Dynamics of Nature; Terborgh, J., Estes, J.A., Eds.; Island Press: Washington, DC, USA, 2010; pp. 319–335. [Google Scholar]
- Hayward, M.W.; Edwards, S.; Fancourt, B.A.; Linnell, J.D.C.; Nilsen, E.B. Top-down control of ecosystems and the case for rewilding: Does it all add up? In Rewilding; Pettorelli, N., Durant, S.M., du Toit, J.T., Eds.; Cambridge University Press: Cambridge, MA, USA, 2018; pp. 325–354. [Google Scholar]
- Boitani, L.; Linnell, J.D.C. Bringing large mammals back: Large carnivores in Europe. In Rewilding European Landscapes; Pereira, H.M., Navarro, L.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 67–84. [Google Scholar]
- Linnell, J.D.C.; Promberger, C.; Boitani, L.; Swenson, J.E.; Breitenmoser, U.; Andersen, R. The linkage between conservation strategies for large carnivores and biodiversity: The view from the “half-full”forests of Europe. In Carnivorous Animals and Biodiversity: Does Conserving One Save the Other? Ray, J.C., Redford, K.H., Steneck, R.S., Berger, J., Eds.; Island Press: Washington, DC, USA, 2005; pp. 381–398. [Google Scholar]




| Intercept | Season | Distance a | K b | AICc | Δ AICc c | ωi d | |
|---|---|---|---|---|---|---|---|
| (a) C/N ratio (log) | 2.95 | 3 | −38.70 | 0.00 | 0.634 | ||
| 2.87 | + | 4 | −37.50 | 1.15 | 0.357 | ||
| 2.94 | 0.01 | 4 | −29.20 | 9.50 | 0.006 | ||
| 2.86 | + | 0.01 | 5 | −28.00 | 10.70 | 0.003 | |
| (b) N (square root) | 0.92 | 3 | −35.40 | 0.00 | 0.895 | ||
| 0.92 | + | 4 | −30.60 | 4.80 | 0.081 | ||
| 0.94 | −0.01 | 4 | −27.90 | 7.47 | 0.021 | ||
| 0.95 | + | −0.01 | 5 | −23.00 | 12.32 | 0.002 | |
| (c) NH4+ (log) | −6.25 | 3 | 120.20 | 0.00 | 0.670 | ||
| −6.14 | + | 4 | 121.90 | 1.74 | 0.281 | ||
| −6.18 | −0.04 | 4 | 126.10 | 5.94 | 0.034 | ||
| −6.08 | + | −0.04 | 5 | 127.90 | 7.73 | 0.014 | |
| (d) NO3− (log) | −9.18 | 3 | 231.90 | 0.00 | 0.565 | ||
| −9.11 | + | 4 | 232.90 | 1.01 | 0.341 | ||
| −9.05 | −0.08 | 4 | 236.40 | 4.50 | 0.060 | ||
| −8.98 | + | −0.08 | 5 | 237.40 | 5.58 | 0.035 | |
| (e) P (cube root) | 0.41 | 3 | −241.60 | 0.00 | 0.884 | ||
| 0.40 | + | 4 | −237.50 | 4.13 | 0.112 | ||
| 0.42 | 0.00 | 4 | −230.30 | 11.29 | 0.003 | ||
| 0.40 | + | 0.00 | 5 | −226.00 | 15.56 | 0.000 | |
| (f) PO4− (log) | −7.60 | + | 4 | 261.90 | 0.00 | 0.491 | |
| −7.91 | 3 | 262.40 | 0.44 | 0.394 | |||
| −7.44 | + | −0.09 | 5 | 266.00 | 4.11 | 0.063 | |
| −7.75 | −0.09 | 4 | 266.40 | 4.49 | 0.052 | ||
| (g) Ca (square root) | 0.69 | 3 | −130.50 | 0.00 | 0.945 | ||
| 0.69 | + | 4 | −124.40 | 6.02 | 0.047 | ||
| 0.70 | −0.01 | 4 | −120.90 | 9.59 | 0.008 | ||
| 0.70 | + | −0.01 | 5 | −114.80 | 15.69 | 0.000 | |
| (h) K (log) | −4.25 | 3 | 145.20 | 0.00 | 0.661 | ||
| −4.14 | + | 4 | 146.80 | 1.57 | 0.301 | ||
| −4.20 | −0.03 | 4 | 151.70 | 6.44 | 0.026 | ||
| −4.09 | + | −0.03 | 5 | 153.30 | 8.07 | 0.012 | |
| (i) Mg (square root) | 0.13 | 3 | −285.60 | 0.00 | 0.983 | ||
| 0.13 | + | 4 | −276.60 | 9.01 | 0.011 | ||
| 0.13 | 0.00 | 4 | −275.30 | 10.27 | 0.006 | ||
| 0.14 | + | 0.00 | 5 | −266.30 | 19.34 | 0.000 | |
| (j) Na (cube root) | 0.19 | 3 | −160.00 | 0.00 | 0.865 | ||
| 0.21 | + | 4 | −156.10 | 3.90 | 0.123 | ||
| 0.20 | −0.01 | 4 | −151.00 | 8.95 | 0.010 | ||
| 0.22 | + | −0.01 | 5 | −147.10 | 12.89 | 0.001 |
| Intercept | Season | Distance | K | AICc | Δ AICc | ωi | |
|---|---|---|---|---|---|---|---|
| (a) C:N ratio (log) | 3.37 | 3 | −5.00 | 0.000 | 0.818 | ||
| 3.33 | + | 4 | −1.90 | 3.140 | 0.170 | ||
| 3.34 | 0.01 | 4 | 3.70 | 8.720 | 0.010 | ||
| 3.29 | + | 0.01 | 5 | 7.00 | 12.090 | 0.002 | |
| (b) N (square root) | 1.29 | 3 | −8.50 | 0.000 | 0.896 | ||
| 1.27 | + | 4 | −3.70 | 4.770 | 0.082 | ||
| 1.35 | −0.02 | 4 | −0.90 | 7.640 | 0.020 | ||
| 1.33 | + | −0.02 | 5 | 4.20 | 12.720 | 0.002 | |
| (c) P (cube root) | 0.49 | 3 | −90.80 | 0.000 | 0.939 | ||
| 0.51 | −0.01 | 4 | −84.90 | 5.940 | 0.048 | ||
| 0.49 | + | 4 | −82.20 | 8.650 | 0.012 | ||
| 0.51 | + | −0.01 | 5 | −75.80 | 15.000 | 0.001 | |
| (d) Ca (square root) | 0.91 | 3 | −59.00 | 0.000 | 0.958 | ||
| 0.90 | + | 4 | −52.40 | 6.590 | 0.035 | ||
| 0.93 | −0.01 | 4 | −49.10 | 9.890 | 0.007 | ||
| 0.92 | + | −0.01 | 5 | −42.20 | 16.790 | 0.000 | |
| (e) K (log) | −0.26 | 3 | 29.50 | 0.000 | 0.868 | ||
| −0.28 | + | 4 | 33.60 | 4.130 | 0.110 | ||
| −0.28 | 0.01 | 4 | 37.00 | 7.570 | 0.020 | ||
| −0.30 | + | 0.01 | 5 | 41.40 | 11.950 | 0.002 | |
| (f) Mg (square root) | 0.46 | 3 | −87.30 | 0.000 | 0.968 | ||
| 0.45 | + | 4 | −80.20 | 7.050 | 0.029 | ||
| 0.47 | 0.00 | 4 | −75.90 | 11.370 | 0.003 | ||
| 0.46 | + | 0.00 | 5 | −68.50 | 18.820 | 0.000 | |
| (g) Na (cube root) | 0.13 | 3 | −27.90 | 0.000 | 0.931 | ||
| 0.15 | + | 4 | −22.40 | 5.470 | 0.060 | ||
| 0.15 | −0.01 | 4 | −18.40 | 9.490 | 0.008 | ||
| 0.17 | + | −0.01 | 5 | −12.70 | 15.200 | 0.000 |
| Intercept | Season | Distance | K | AICc | Δ AICc | ωi | |
|---|---|---|---|---|---|---|---|
| (a) C/N ratio (log) | 3.42 | 3 | 6.30 | 0.000 | 0.903 | ||
| 3.40 | + | 4 | 11.30 | 4.940 | 0.076 | ||
| 3.36 | 0.02 | 4 | 14.10 | 7.730 | 0.019 | ||
| 3.34 | + | 0.02 | 5 | 19.40 | 13.060 | 0.001 | |
| (b) N (square root) | 1.20 | 3 | −4.50 | 0.000 | 0.935 | ||
| 1.20 | + | 4 | 1.30 | 5.800 | 0.051 | ||
| 1.17 | 0.01 | 4 | 4.00 | 8.530 | 0.013 | ||
| 1.16 | + | 0.01 | 5 | 10.10 | 14.570 | 0.001 | |
| (c) P (cube root) | 0.50 | 3 | −59.20 | 0.000 | 0.938 | ||
| 0.49 | + | 4 | −53.60 | 5.570 | 0.058 | ||
| 0.49 | 0.00 | 4 | −48.20 | 10.920 | 0.004 | ||
| 0.48 | + | 0.00 | 5 | −42.20 | 16.970 | 0.000 | |
| (d) Ca (square root) | 0.36 | 3 | −53.80 | 0.000 | 0.948 | ||
| 0.34 | + | 4 | −47.80 | 5.960 | 0.048 | ||
| 0.36 | 0.00 | 4 | −42.70 | 11.080 | 0.004 | ||
| 0.35 | + | 0.00 | 5 | −36.50 | 17.240 | 0.000 | |
| (e) K (log) | 0.73 | 3 | 16.20 | 0.000 | 0.894 | ||
| 0.74 | + | 4 | 20.80 | 4.620 | 0.089 | ||
| 0.69 | 0.01 | 4 | 24.30 | 8.100 | 0.016 | ||
| 0.71 | + | 0.01 | 5 | 29.10 | 12.960 | 0.001 | |
| (f) Mg (square root) | 0.30 | 3 | −56.80 | 0.000 | 0.976 | ||
| 0.30 | + | 4 | −48.80 | 7.970 | 0.018 | ||
| 0.32 | −0.01 | 4 | −46.50 | 10.280 | 0.006 | ||
| 0.32 | + | −0.01 | 5 | −38.20 | 18.640 | 0.000 | |
| (g) Na (cube root) | 0.11 | 3 | 23.00 | 0.000 | 0.923 | ||
| 0.14 | + | 4 | 17.60 | 5.380 | 0.063 | ||
| 0.15 | −0.01 | 4 | 14.50 | 8.460 | 0.013 | ||
| 0.17 | + | −0.01 | 5 | −8.70 | 14.300 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teurlings, I.J.M.; Melis, C.; Skarpe, C.; Linnell, J.D.C. Lack of Cascading Effects of Eurasian Lynx Predation on Roe Deer to Soil and Plant Nutrients. Diversity 2020, 12, 352. https://doi.org/10.3390/d12090352
Teurlings IJM, Melis C, Skarpe C, Linnell JDC. Lack of Cascading Effects of Eurasian Lynx Predation on Roe Deer to Soil and Plant Nutrients. Diversity. 2020; 12(9):352. https://doi.org/10.3390/d12090352
Chicago/Turabian StyleTeurlings, Ivonne J. M., Claudia Melis, Christina Skarpe, and John D. C. Linnell. 2020. "Lack of Cascading Effects of Eurasian Lynx Predation on Roe Deer to Soil and Plant Nutrients" Diversity 12, no. 9: 352. https://doi.org/10.3390/d12090352
APA StyleTeurlings, I. J. M., Melis, C., Skarpe, C., & Linnell, J. D. C. (2020). Lack of Cascading Effects of Eurasian Lynx Predation on Roe Deer to Soil and Plant Nutrients. Diversity, 12(9), 352. https://doi.org/10.3390/d12090352