Frustulia tunariensis sp. nov. (Bacillariophyceae) from the Andes of Bolivia, South America
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection, Treatment and Analysis
3. Results
3.1. Accompanying Flora and Diatom Count
3.2. New Species Description
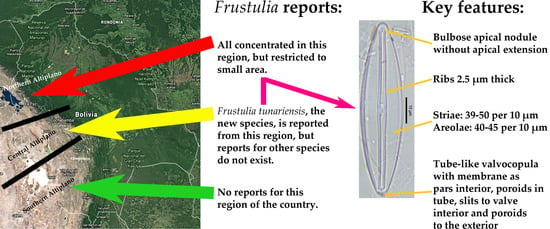
3.3. Distribution of Frustulia Species in the Bolivian Altiplano
4. Discussion
4.1. Taxonomic Allocation and Distinction of Frustulia tunariensis sp. nov. from Similar Species
4.2. Ecology of F. tunariensis sp. nov.
4.3. Use of Girdle Bands to Characterize Frustulia spp.
4.4. Distribution of Frustulia spp. from the Bolivian Altiplano
Funding
Acknowledgments
Conflicts of Interest
References
- Morales, E.A.; Novais, M.H.; Chávez, G.; Hoffmann, L.; Ector, L. Diatoms (Bacillariophyceae) from the Bolivian Altiplano: Three new araphid species from the Desaguadero River draining Lake Titicaca. Fottea 2012, 12, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Morales, E.A.; Wetzel, C.E.; Rivera, S.F.; Van de Vijver, B.; Ector, L. Current taxonomic studies on the diatom flora (Bacillariophyceae) of the Bolivian Altiplano, South America, with possible consequences for palaeoecological assessments. J. Micropalaeontol. 2014, 33, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Frenguelli, J. Diatomeas del Lago Titicaca. Notas Mus. Plata Bot. 1939, 24, 175–196. [Google Scholar]
- Servant-Vildary, S. Altitudinal zonation of mountainous diatom flora in Bolivia: Application to the study of the Quaternary. Acta Geol. Acad. Sci. Hung. 1982, 25, 179–210. [Google Scholar]
- Servant-Vildary, S. Les diatomées actuelles des Andes de Bolivie (Taxonomie, écologie). Cah. Micropaléontol. 1986, 1, 99–124. [Google Scholar]
- Servant-Vildary, S. The diatoms. In Lake Titicaca; Dejoux, C., Iltis, A., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1991; pp. 163–175. [Google Scholar]
- Metzeltin, D.; Lange-Bertalot, H. Tropical diatoms of South America I. About 700 predominantly rarely known or new taxa representative of the neotropical flora. Iconogr. Diatomol. 1998, 5, 695. [Google Scholar]
- Rumrich, U.; Lange-Bertalot, H.; Rumrich, M. Diatomeen der Anden von Venezuela bis Patagonien/Feuerland. Iconogr. Diatomol. 2000, 9, 1–649. [Google Scholar]
- Tapia, P.M.; Theriot, E.C.; Fritz, S.C.; Cruces, F.; Rivera, P. Distribution and morphometric analysis of Cyclostephanos andinus comb. nov., a planktonic diatom from the central Andes. Diatom Res. 2004, 19, 311–327. [Google Scholar] [CrossRef]
- Van de Vijver, B.; Cocquyt, C. Four new diatom species from La Calera Hot Spring in the Peruvian Andes (Colca Canyon). Diatom Res. 2009, 29, 209–223. [Google Scholar] [CrossRef]
- Álvarez-Blanco, I.; Cejudo-Figueiras, C.; de Godos, I.; Muñoz, R.; Blanco, S. Las diatomeas de los salares del Altiplano boliviano: Singularidades florísticas. Bol. Real Soc. Esp. Hist. Nat. Secc. Biol. 2011, 105, 67–82. [Google Scholar]
- Blanco, S.; Álvarez-Blanco, I.; Cejudo-Figueiras, C.; de Godos, I.; Bécares, E.; Muñoz, R.; Guzman, H.O.; Vargas, V.A.; Soto, R. New diatom taxa from high-altitude Andean saline lakes. Diatom Res. 2013, 28, 13–27. [Google Scholar] [CrossRef]
- Furey, P.C.; Mayama, S.; Lowe, R.L.; Catenazzi, A. Frankophila wayqechae sp. nov., a new aerophilic diatom species from the Peruvian Andes, South America. Diatom Res. 2012, 27, 165–175. [Google Scholar] [CrossRef]
- Morales, E.A.; Rivera, S.F.; Wetzel, C.E.; Novais, M.H.; Hamilton, P.B.; Hoffmann, L.; Ector, L. New epiphytic araphid diatoms in the genus Ulnaria (Bacillariophyta) from Lake Titicaca, Bolivia. Diatom Res. 2014, 29, 41–54. [Google Scholar] [CrossRef]
- Seeligmann, C.T.; Maidana, N.I.; Morales, E.A. Fragilariaceae (Bacillariophyta) en humedales de altura de Catamarca (Argentina). Bol. Soc. Argent. Bot. 2018, 53, 507–519. [Google Scholar] [CrossRef] [Green Version]
- Lange-Bertalot, H.; Jahn, R. On the identity of Navicula (Frustulia) rhomboides and Frustulia saxonica (Bacillariophyceae). Syst. Geogr. Plants 2000, 70, 255–261. [Google Scholar] [CrossRef]
- Metzeltin, D.; Lange-Bertalot, H. Tropical diatoms of South America II. Special remarks on biogeographic disjunction. Iconogr. Diatomol. 2007, 18, 1–877. [Google Scholar]
- Metzeltin, D.; Lange-Bertalot, H.; García-Rodríguez, F. Diatoms of Uruguay compared with other taxa from South America and elsewhere. Iconogr. Diatomol. 2005, 15, 1–736. [Google Scholar]
- Soares, F.S.; Konoplya, B.I.B.; Da Silva, J.F.M.; de Andrade, C.G.T.J. Amphipleuraceae (Bacillariophyceae) do alto da bacia do Ribeirão Cambé, Londrina, Brasil. Rev. Bras. Bot. 2011, 34, 39–49. [Google Scholar] [CrossRef]
- Casa, V.; Mataloni, G.; Van de Vijver, B. Six new Frustulia species (Bacillariophyta) in Tierra del Fuego peatbogs, Patagonia, Argentina. Fottea 2018, 18, 55–71. [Google Scholar] [CrossRef]
- Brassac, N.M.; Ludwig, T.A.V. Amphipleuraceae e Diploneidaceae (Bacillariophyceae) da bacia do rio Iguaçu, PR, Brasil. Acta Bot. Bras. 2005, 19, 359–368. [Google Scholar] [CrossRef]
- Benito, X.; Fritz, S. Diatom diversity and biogeography across tropical South America. In Neotropical Diversification: Patterns and Processes; Rull, V., Carnaval, A.C., Eds.; Springer Nature: Geneva, Switzerland, 2020; Chapter 7; pp. 121–143. [Google Scholar]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms: Biology & Morphology of the Genera; Cambridge University Press: Cambridge, MA, USA, 1990; p. 747. [Google Scholar]
- Lange-Bertalot, H. Navicula sensu stricto. 10 genera separated from Navicula sensu lato, Frustulia. Diatoms Eur. 2001, 2, 1–256. [Google Scholar]
- Cox, E.J. Variation in patterns of valve morphogenesis between representatives of six biraphid diatom genera (Bacillariophyceae). J. Phycol. 1999, 35, 1297–1312. [Google Scholar] [CrossRef]
- Patrick, R.; Reimer, C.W. The diatoms of the United States I. Monogr. Acad. Nat. Sci. Phila. 1966, 13, 1–688. [Google Scholar]
- Graeff, C.L.; Kociolek, J.P.; Burliga, A.L. Valve morphology of four species of Frustulia (Bacillariophyta), including two described as new. Phytotaxa 2012, 42, 62–76. [Google Scholar] [CrossRef]
- Siver, P.; Baskette, G. A morphological examination of Frustulia (Bacillariophyceae) from the Ocala National Forest, Florida, USA. Can. J. Bot. 2004, 82, 629–644. [Google Scholar] [CrossRef]
- Sawai, Y.; Magumo, T.; Nelson, A.R. A brackish diatom, Pseudofrustulia lancea gen. et sp. nov. (Bacillariophyceae) from the Pacific coast of Oregon (USA). Phytotaxa 2016, 267, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Montes de Oca, I. Geografía y Recursos Naturales de Bolivia, 3rd ed.; EDOBOL: La Paz, Bolivia, 1997; p. 614. [Google Scholar]
- Bicudo, C.E.d.M.; Menezes, M. Gêneros de Algas de Águas Continentais no Brasil, 3rd ed.; Rima Editora: Sâo Paulo, Brasil, 2017; p. 552. [Google Scholar]
- Manguin, E. Contribution à la connaissance des diatomées des Andes du Pérou. Mém. Mus. Natl. Hist. Nat. B Bot. 1964, 12, 41–98. [Google Scholar]
- Morales, E.A.; Vis, M.L. Epilithic diatoms (Bacillariophyceae) from cloud forest and alpine streams in Bolivia, South America. Proc. Acad. Nat. Sci. Phila. 2007, 156, 123–155. [Google Scholar] [CrossRef]
- Morales, E.A.; Vis, M.L.; Férnandez, E.; Kociolek, J.P. Epilithic diatoms (Bacillariophyta) from cloud forest and alpine streams in Bolivia, South America II: A preliminary report on the diatoms from Sorata, Department of La Paz. Acta Nova 2007, 3, 680–696. [Google Scholar]
- Hohn, M.H. Bacillariophyta. Monogr. Acad. Nat. Sci. Phila. 1966, 14, 459–495. [Google Scholar]
- Krammer, K. Die cymbelloiden Diatomeen. Eine Monographie der weltweit bekannten Taxa. Teil 1. Allgemeines und Encyonema Part. Bibl. Diatomol. 1997, 36, 1–382. [Google Scholar]
- Krammer, K. Die cymbelloiden Diatomeen. Eine Monographie der weltweit bekannten Taxa. Teil 2. Encyonema part, Encyonopsis and Cymbellopsis. Bibl. Diatomol. 1997, 37, 1–469. [Google Scholar]
- Krammer, K. The genus Pinnularia. In Diatoms of Europe. Diatoms of the European Inland Waters and Comparable Habitats; Lange-Bertalot, H., Ed.; Gantner Verlag: Ruggell, Liechtenstein, 2000; Volume 1, pp. 1–703. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 1. Teil: Naviculaceae. In Süsswasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1986; Volume 2, pp. 1–876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Süsswasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1988; Volume 2/2, pp. 1–596. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Süsswasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart/Jena, Germany, 1991; Volume 2/3, pp. 1–576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. Gesamtliteraturverzeichnis Teil 1–4. In Süsswasserflora von Mitteleuropa; Ettl, H., Gärtner, G., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart/Jena, Germany, 1991; Volume 2/4, pp. 1–437. [Google Scholar]
- Lange-Bertalot, H. 85 Neue Taxa und über 100 weitere neu defi nierte Taxa ergänzend zur Süsswasserfl ora von Mitteleuropa Vol. 2/1-4. Bibl. Diatomol. 1993, 27, 1–454. [Google Scholar]
- Lange-Bertalot, H.; Moser, G. Brachysira Monographie der Gattung. Wichtige Indikator-Species für das Gewässer-Monitoring und Naviculadicta nov. gen. Ein Lösungsvorschlag zu dem Problem Navicula sensu lato ohne Navicula sensu stricto. Bibl. Diatomol. 1994, 29, 1–212. [Google Scholar]
- Battarbee, R.W. Diatom analysis. In Handbook of Holocene Palaeoecology and Palaeohydrology; Berglund, B.E., Ed.; John Wiley and Sons: Chichester, UK, 1986; pp. 527–570. [Google Scholar]
- Kociolek, J.P.; Graeff, C.L.; Thomas, E.W. A description of the frustular morphology of Frustulia creuzburgensis (Krasske) Hustedt, with comments on its systematic position. Diatom Res. 2011, 26, 29–41. [Google Scholar] [CrossRef]
- Moser, G.; Steindorf, A.; Lange-Bertalot, H. Newkaledonien. Diatomeenflora einer Tropeninsel Revision der Collection Maillard und Untersuchung neuen Materials. Bibl. Diatomol. 1995, 32, 1–340. [Google Scholar]
- Edlund, M.B.; Brant, L.A. Frustulia Bahlsii sp. nov., a freshwater diatom from the Eastern U.S.A. Diatom Res. 1997, 12, 207–216. [Google Scholar] [CrossRef]
- Bourrelly, P.; Manguin, É. Algues D’eau Douce de la Guadeloupe et Dépendances: Recueillies par la Mission P. Allorge en 1936; Société d’Édition d’Enseignement Superiéur: Paris, France, 1952; pp. 1–282. [Google Scholar]
- Da Silva-Lehmkuhl, A.M.; Tremarin, P.I.; Vercellino, I.S.; Ludwig, T.A.V. Periphytic diatoms from an oligotrophic lentic system, Piraquara I reservoir, Paraná state, Brazil. Biota Neotrop. 2019, 19, e20180568. [Google Scholar] [CrossRef]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar]
- Noga, T.; Peszek, Ł.; Stanek-Tarkowska, J.; Pajączek, A. The Pinnularia genus in south-eastern Poland with consideration or rare and taxa to Poland. Oceanol. Hydrobiol. Stud. 2014, 43, 77–99. [Google Scholar] [CrossRef]
- Graeff, C.L.; Kociolek, J.P. Investigations of the Frustulia weinholdii species complex with descriptions of three new species, F. capitata, F. latita, and F. soror from South Carolina and Hawaii. Proc. Acad. Nat. Sci. Phila. 2011, 161, 43–59. [Google Scholar] [CrossRef]
- Navarro, G.; Maldonado, M. Geografía Ecológica de Bolivia. Vegetación y Ambientes Acuáticos, 5th ed.; Fundación Simón I. Patiño: Cochabamba, Bolivia, 2011; p. 719. [Google Scholar]
- Navarro, G.; De la Barra, N.; Goitia, E.; Maldonado, M. Propuesta metodológica para la clasificación de humedales altoandinos en Bolivia. Rev. Boliv. Ecol. Conserv. Ambient. 2011, 29, 1–22. [Google Scholar]





| Taxon Name | Valve Count | Percentage |
|---|---|---|
| Achnanthidium cf. nanum (F. Meister) Novais & I. Jüttner | ||
| Achnanthidium minutissimum (Kützing) Czarnecki | 2 | 0.5 |
| Chammaepinnularia sp. 1 | ||
| Cymbopleura naviculiformis (Auerswald) Krammer | ||
| Diploneis kahlii Lange-Bertalot & Rumrich | ||
| Encyonema aueri (Krasske) Krammer | 2 | 0.5 |
| Encyonema dubium Krammer | ||
| Encyonema minutiforme Krammer | ||
| Encyonema neogracile Krammer | 26 | 6.5 |
| Encyonema rumrichae Krammer | 4 | 1 |
| Encyonema silesiacum (Bleisch) D.G. Mann | 2 | 0.5 |
| Encyonopsis cf. ruttneri (Hustedt) Krammer | 15 | 3.75 |
| Encyonopsis cf. thumensis Krammer | 45 | 11.25 |
| Encyonopsis recta Krammer | ||
| Encyonopsis sp. 1 | 10 | 2.5 |
| Eunotia cf. biggiba Kützing | ||
| Eunotia cf. major (W. Smith) Rabenhorst | ||
| Eunotia parapectinalis Lange-Bertalot & U. Rumrich | ||
| Fragilaria rumpens (Kützing) Carlson | ||
| Fragilaria tenera (W. Smith) Lange-Bertalot | 22 | 5.5 |
| Frustulia saxonica Rabenhorst | 1 | 0.25 |
| Frustulia tunariensis sp. nov. | 30 | 7.5 |
| Gomphonema cf. gracile Ehrenberg | ||
| Gomphonema cf. parvulum (Kützing) Kützing | ||
| Gomphonema cf. subclavatum (Grunow) Grunow | ||
| Kobayasiella pseudosubtilissima (Manguin) Lange-Bertalot & E.Reichardt | 23 | 5.75 |
| Krasskella sp. 1 | ||
| Neidium cf. bisulcatum (Lagerstedt) Cleve | ||
| Nitzschia cf. acidoclinata Lange-Bertalot | 70 | 17.5 |
| Nitzschia cf. perminuta Grunow | 106 | 26.5 |
| Nitzschia cf. subacicularis Hustedt | 2 | 0.5 |
| Nitzschia cf. strelnikovae Lange-Bertalot, Genkal & Vekhov | ||
| Nitzschia neotropica Lange-Bertalot & U. Rumrich | 2 | 0.5 |
| Pinnularia borealis Ehrenberg | ||
| Pinnularia borealis var. islandica Krammer | 2 | 0.5 |
| Pinnularia cf. ampulliformis Manguin | 7 | 1.75 |
| Pinnularia divergentissima Grunow | ||
| Pinnularia microstauron (Ehrenberg) Cleve | 14 | 3.5 |
| Pinnularia pseudogibba Krammer | ||
| Pinnularia rabenhorstii var. franconica Krammer | ||
| Pinnularia similiformis Krammer | ||
| Pinnularia spinosissima Lange-Bertalot, Rumrich & Krammer | 2 | 0.5 |
| Pinnularia subcapitata var. elongata Krammer | 5 | 1.25 |
| Pinnularia subgibba Krammer | ||
| Pinnularia tsoneka Lange-Bertalot & Metzeltin | ||
| Pseudostaurosira sp. 1 | ||
| Rhopalodia cf. operculata (C.Agardh) Håkanasson | ||
| Sellaphora fusticulus (Østrup) Lange-Bertalot | 2 | 0.5 |
| Stauroneis acidoclinata Lange-Bertalot & Werum | ||
| Stauroneis cf. frauenfeldianum (Grunow) Heiden | ||
| Stauroneis subgracilis Lange-Bertalot & Krammer | ||
| Stenopterobia cf. delicatissima (F.W.Lewis) Brébisson ex Van Heurck | ||
| Stenopterobia densistriata (Hustedt) Krammer | ||
| Tabellaria flocculosa (Roth) Kützing | 5 | 1.25 |
| Ulnaria acus (Kützing) Aboal | 1 | 0.25 |
| Ulnaria sp. 1 | ||
| TOTAL | 400 | 100 |
| Taxa | Locality and Altitude (m a.s.l.) | Coordinates | General Ecology | Reference & Comments |
|---|---|---|---|---|
| F. corneliae Lange-Bertalot & U. Rumrich | Small unnamed stream in Sorata, La Paz, 4056 | 15°42′0.35″ S, 68°36′0.41″ W | Rare, in stream benthos, circumneutral to alkalibiont | [34], as “F. sp. 1”, only LM |
| F. crassinervia (Brébisson ex Smith) Lange-Bertalot & Krammer | Small unnamed stream in Sorata, La Paz, 4056 | 15°42′0.35″ S, 68°36′0.41″ W | Rare, in stream benthos, alkalibiont | [34], only LM |
| F. franguelli Manguin | Ichu Kota Valley, La Paz, 4200–4900 | 16°12′ S, 69°30′ W | Rare, in high altitude peatland, acidobiontic | [4,5], only listed |
| F. rhomboides (Ehrenberg) De Toni | Ichu Kota Valley, La Paz, 4200–4900 | 16°12′ S, 69°30ʹ W | Rare, in high altitude peatland, acidobiontic | [4,5], only listed |
| F. saxonica (Thwaites) De Toni | -Ichu Kota Valley, La Paz, 4200–4900 -Peatland near town of Uyuni, Cochabamba, 4155 | −16°12′ S, 69°30ʹ W 17°7′13.1″ S, 66°17′51″ W | Rare, in high altitude peatlands, acidobiontic | [4,5], only listed -This manuscript, only LM (Figure 3a´,b´) |
| F. tunariensis E. Morales sp. nov. | Peatland near town of Uyuni, Cochabamba, 4155 | 17°7′13.1″S, 66°17′51″W | Common, in high altitude peatland, acidobiontic | This manuscript, LM and SEM available |
| F. vulgaris (Thwaites) De Toni | -Two small unnamed streams in Sorata, La Paz 3993–4056 -Lake Titicaca, La Paz, 3812 | −15°42′0.35″ S, 68°36′0.41″ W; 15°51′2.47″ S, 68°38′6.39″ W −15°45′ S, 69°25′ W | Rare, in lake and stream benthos, circumneutral to alkalibiont | [6,34], only listed |
| Taxon/Features | Valve Shape | Valve Dimensions (µm)/Areola and Stria Density (in 10 µm), and Orientation | Raphe Endings | Longitudinal Ribs/Helictoglossa Complex | Comment/Reference |
|---|---|---|---|---|---|
| F. altimontana Metzeltin & Lange-Bertalot | Lanceolate with obtusely to broadly rounded apices | Length: 115–145 Width: 22–27 Areolae: 15–19 Striae: 22–24, parallel to convergent; circumradial at apices | Unknown | Ribs ca. 1.3 µm thick. Helictoglossa unknown. Apical nodule non-bulbose, but unclear from LM; apical extension unclear. Central nodule biconvex | Only known from LM [7] |
| F. bahlsii Edlund & Brant | Lanceolate to rhombic-lanceolate with broadly rounded ends, sometimes rostrate | Length: 98–193 Width: 24–33 Areolae: 16–23 Striae: 20–26, parallel; slightly circumparallel to circumradial at apices | Externally, proximal and distal ends T-shaped. Internally, the distal and proximal terminations straight | Ribs ca. 0.8 µm thick. Helictoglossa inconspicuous and porte-crayon structure very low. Apical nodule notably bulbose without an apical extension. Central nodule constricted | [28,48] |
| F. chilensis Lange-Bertalot & Rumrich | Elliptico-lanceolate with short, rostrate, widely rounded apices | Length: 94–110 Width: 20–24 Areolae: 26–29 Striae: 20–27, parallel to subparallel; unknown pattern at apices | Unknown | Ribs ca. 1.3 µm thick. Helictoglossa unknown. Polar nodule bulbose with very short apical extension. Central nodule diffuse and unclear | Only known from LM [8] |
| F. blancheana Maillard | Lanceolate with subrostrate, broadly rounded apices | Length: 70–84 Width: 10–13 Areolae: 25–30 Striae: 28–33, parallel to slightly convergent; circumradiate around apices | Externally, proximal ends slightly deflected in the same direction, distal ends straight. Internally, the distal and proximal terminations straight | Ribs ca. 1.7 µm thick. Helictoglossa a relatively high porte-crayon structure. Apical nodule notably bulbose with long apical extension. Central nodule constricted | [47] |
| F. crassiareolaeta Metzeltin & Lange-Bertalot | Lanceolate with faintly subrostrate, broadly rounded apices | Length: 77–100 Width: 17.5–21 Areolae: 20–22 Striae: 23–26.5, parallel to subparallel; circumradiate around apices | Unknown | Ribs ca. 1 µm thick. Helictoglossa unknown. Polar nodule non-bulbose with long apical extension. Central nodule irregular, sometimes constricted | Only known from LM [7] |
| F. crassiareolaetoides Metzeltin & Lange-Bertalot | Lanceolate with broadly rounded apices | Length: 77–115 Width: 17–23 Areolae: 20–22 Striae: 24–26, parallel to subparallel; circumradiate around apices | Unknown | Ribs ca. 1 µm thick. Helictoglossa unknown. Apical nodule bulbose with large apical extension. Central nodule highly constricted | Only known from LM [17] |
| F. ellipticolanceolata Casa, Mataloni & Van de Vijver | Lanceolate with subrostrate ends | Length: 51–69 Width: 12.5–15.5 Areolae: 25–35 Striae: 30–32, parallel at center, weakly convergent toward ends; circumradiate around apices, sometimes reduced to a single apical areola per apical stria. | Externally, proximal and distal ends T-shaped. Internally, the distal and proximal terminations straight | Ribs up to ca. 0.9 µm thick. Helictoglossa a relatively high porte-crayon structure. Apical nodule bulbose with apical extension. Central nodule constricted | [20] |
| F. fuegiana Casa, Mataloni & Van de Vijver | Lanceolate with faint subrostrate to bluntly cuneate ends. | Length: 44–90 Width: 12–17 Areolae: 24–28 Striae: 26–29, parallel at center becoming weakly convergent toward ends | Externally, proximal and distal ends T-shaped. Internally, the distal and proximal terminations straight | Ribs up to ca. 0.8 µm thick. Helictoglossa a faint porte-crayon structure. Apical nodule faintly bulbose. Central nodule weakly to noticeably constricted | [20] |
| F. magna Metzeltin & Lange-Bertalot | Lanceolate to rhombic-lanceolate with broadly rounded ends | Length: 130–160 Width: 31–33 Areolae: 18–20 Striae: 20–22, parallel to convergent; circumradiate around apices | Unknown | Ribs ca. 1.7 µm thick. Helictoglossa unknown. Apical nodule non-bulbose with large apical extension. Central nodule slightly biconvex to highly constricted | Only known from LM [7] |
| F. neofrenguellii Lange-Bertalot & Rumrich | Elliptico-lanceolate, with short subrostrate and obstusely rounded apices | Length: 55–80 Width: 16–18 Areolae: 24–26 Striae: 24–26, parallel; circumradiate around apices | Externally, proximal and distal ends T-shaped. Internally, the distal terminations end in a small punctum; proximal ends unknown | Ribs ca. 1 µm thick. Helictoglossa, a low porte-crayon structure forming a short apical extension on a non-bulbose polar nodule. Central nodule constricted and only known in LM. | SEM information incomplete [8] |
| F. pangaeopsis Lange-Bertalot | Elliptico-lanceolate with rostrate to subcapitate, widely rounded apices | Length: 75–90 Width: 16–18.5 Areolae: 24–26 Striae: 6–27, parallel to slightly convergent; circumradiate around apices | Externally, proximal and distal ends unknown. Internally, the distal terminations straight; proximal ends unknown | Ribs ca. 0.7 µm thick. Helictoglossa, inconspicuous and porte-crayon structure faint, forming an also faint short apical extension on a slightly bulbose polar nodule. Central nodule constricted and only known in LM. | SEM information incomplete [24] |
| F. tunariensis E. Morales sp. nov. | Elliptico-lanceolate with obtusely rounded apices | Length: 60–110 Width: 15.5–18 Areolae: 40–45 Striae: 39–50, parallel to convergent, circumradiate around apices | Externally, proximal and distal ends T-shaped. Internally, the distal and proximal terminations straight | Ribs ca. 2.5 µm thick. Helictoglossa inconspicuous and porte-crayon structure faint. Apical nodule notably bulbose without an apical extension. Central nodule irregular, sometimes constricted | This manuscript |
| F. turfosa Metzeltin & Lange-Bertalot | Lanceolate with subrostrate, broadly rounded apices | Length: 100–150 Width: 16.6–19 Areolae: 18–21 Striae: 27, parallel to convergent, circumradiate around apices | Unknown | Ribs ca. 1 µm thick. Helictoglossa unknown. Apical nodule bulbose without an apical extension. Central nodule constricted | Only known from LM [17] |
| Taxon/Features | Copula | Valvocopula | Openings in Valvocopula | Notch and Verrucae on Valvocopula | Reference |
|---|---|---|---|---|---|
| F. blancheana Maillard | Open, with one row of poroids | Open, without fimbriae | Slits in tube-like portion opening as slits toward the interior and as a single row of poroids toward valve exterior | Present, absent | [47] |
| F. creuzburgensis (Krasske) Hustedt | Open, with two rows of poroids | Closed, bearing fimbria | With two rows of poroids in tube-like portion, apparently not open to either valve interior or exterior | Present, verrucae alternate with poroids | [46] |
| F. cf. krammeri | Unknown | Open, lacking fimbriae, but with a flap (pars interior) running on top of the tube-like portion | With one row of slits on the longitudinal flap and one row of slits in the tube-like portion, presumably opening as slits toward the valve interior and not opening towards the valve exterior | Present, verrucae randomly distributed | [27] |
| F. latita Graeff & Kociolek | Open with one or two rows of poroids | Closed, fimbriae undetermined | Poroids inside tube-like portion, opening toward the valve interior undetermined, opening as single row of slits towards the valve exterior becoming a row of poroids toward the valve apices | Present, verrucae alternate with poroids | [43] |
| F. rhomboides (Ehrenberg) De Toni | Open with several rows of poroids | Open, lacking fimbriae, but with an unornamented flap (pars interior) running on top of the tube-like portion | Slits in tube-like portion, open as poroids towards the valve interior and as slits towards the exterior | Present, absent | [23,47] |
| F. tunariensis sp. nov. | Open with one row of poroids | Open, lacking fimbriae but with siliceous membrane on pars interior | Poroids in tube-like portion, open as slits to the interior and as single row of poroids towards the valve exterior | Present, absent | This manuscript |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, E.A. Frustulia tunariensis sp. nov. (Bacillariophyceae) from the Andes of Bolivia, South America. Diversity 2020, 12, 362. https://doi.org/10.3390/d12090362
Morales EA. Frustulia tunariensis sp. nov. (Bacillariophyceae) from the Andes of Bolivia, South America. Diversity. 2020; 12(9):362. https://doi.org/10.3390/d12090362
Chicago/Turabian StyleMorales, Eduardo A. 2020. "Frustulia tunariensis sp. nov. (Bacillariophyceae) from the Andes of Bolivia, South America" Diversity 12, no. 9: 362. https://doi.org/10.3390/d12090362
APA StyleMorales, E. A. (2020). Frustulia tunariensis sp. nov. (Bacillariophyceae) from the Andes of Bolivia, South America. Diversity, 12(9), 362. https://doi.org/10.3390/d12090362