Deer Exclusion Changes Vegetation Structure and Hunting Guilds of Spiders, but Not Multitrophic Understory Biodiversity
Abstract
:1. Introduction
2. Materials and Methods
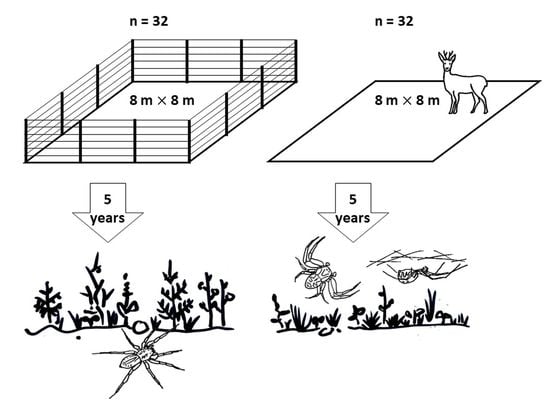
2.1. Study Area
2.2. Field Methods
2.3. Data Analysis
3. Results
3.1. Plant Diversity and Vegetation Structure
3.2. Herbivorous Insect and Spider Abundance, Richness, and Diversity
3.3. Spider Hunting Guilds
4. Discussion
4.1. Plant Diversity and Vegetation Structure
4.2. Arthropod Abundance and Diversity
4.3. Spider Hunting Guilds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forchhammer, M.; Stenseth, N.C.; Post, E.; Langvatn, R. Population dynamics of Norwegian red deer: Density–dependence and climatic variation. Proc. R. Soc. B Boil. Sci. 1998, 265, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic Downgrading of Planet Earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Ripple, W.J.; Estes, J.A.; Beschta, R.L.; Wilmers, C.C.; Ritchie, E.G.; Hebblewhite, M.; Berger, J.; Elmhagen, B.; Letnic, M.; Nelson, M.P.; et al. Status and Ecological Effects of the World’s Largest Carnivores. Science 2014, 343, 1241484. [Google Scholar] [CrossRef] [Green Version]
- Apollonio, M.; Andersen, R.; Putman, R. European Ungulates and Their Management in the 21st Century, 1st ed.; Cambridge Uni-versity Press: New York, NY, USA, 2010. [Google Scholar]
- Burbaitė, L.; Csányi, S. Roe deer population and harvest changes in Europe. Estonian J. Ecol. 2009, 58, 169. [Google Scholar] [CrossRef]
- Côté, S.D.; Rooney, T.P.; Tremblay, J.-P.; Dussault, C.; Waller, D.M. Ecological Impacts of Deer Overabundance. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 113–147. [Google Scholar] [CrossRef] [Green Version]
- Bernes, C.; Macura, B.; Jonsson, B.-G.; Junninen, K.; Müller, J.; Sandström, J.; Lõhmus, A.; Macdonald, E. Manipulating ungulate herbivory in temperate and boreal forests: Effects on vegetation and invertebrates. A systematic review. Environ. Evid. 2018, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Hermy, M.; Verheyen, K. Legacies of the past in the present-day forest biodiversity: A review of past land-use effects on forest plant species composition and diversity. Ecol. Res. 2007, 22, 361–371. [Google Scholar] [CrossRef]
- Huffman, D.; Laughlin, D.C.; Pearson, K.M.; Pandey, S. Effects of vertebrate herbivores and shrub characteristics on arthropod assemblages in a northern Arizona forest ecosystem. For. Ecol. Manag. 2009, 258, 616–625. [Google Scholar] [CrossRef]
- Marquis, R.J. The role of herbivores in terrestrial trophic cascades. In Trophic Cascades. Predators, Prey, and the Changing Dy-namics of Nature; Terborgh, J., Estes, J.A., Eds.; Island Press: Washington, DC, USA, 2010; pp. 109–123. [Google Scholar]
- Kuijper, D.P.; Cromsigt, J.P.; Jędrzejewska, B.; Miścicki, S.; Churski, M.; Jędrzejewski, W.; Kweczlich, I. Bottom-up versus top-down control of tree regeneration in the Białowieża Primeval Forest, Poland. J. Ecol. 2010, 98, 888–899. [Google Scholar] [CrossRef]
- Huffman, D.W.; Moore, M.M. Ungulate Herbivory on Buckbrush in an Arizona Ponderosa Pine Forest. J. Range Manag. 2003, 56, 358. [Google Scholar] [CrossRef]
- Bubnicki, J.W.; Churski, M.; Schmidt, K.; Diserens, T.A.; Kuijper, D.P. Linking spatial patterns of terrestrial herbivore community structure to trophic interactions. eLife 2019, 8, 44937. [Google Scholar] [CrossRef]
- Van Klink, R.; Van Der Plas, F.; Van Noordwijk, C.G.E.; WallisDeVries, M.F.; Olff, H. Effects of large herbivores on grassland arthropod diversity. Biol. Rev. 2014, 90, 347–366. [Google Scholar] [CrossRef] [Green Version]
- Gill, R.; Beardall, V. The impact of deer on woodlands: The effects of browsing and seed dispersal on vegetation structure and composition. For. An Int. J. For. Res. 2001, 74, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Stewart, A. The impact of deer on lowland woodland invertebrates: A review of the evidence and priorities for future research. For. An Int. J. For. Res. 2001, 74, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Suominen, O.; Danell, K. Effects of large herbivores on other fauna. In Large Herbivore Ecology, Ecosystem Dynamics and Con-servation; Danell, K., Bergström, R., Duncan, P., Pastor, J., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 383–412. [Google Scholar]
- Foster, C.N.; Barton, P.S.; Lindenmayer, D.B. Effects of large native herbivores on other animals. J. Appl. Ecol. 2014, 51, 929–938. [Google Scholar] [CrossRef]
- Gómez, J.M.; González-Megías, A. Long-term effects of ungulates on phytophagous insects. Ecol. Entomol. 2007, 32, 229–234. [Google Scholar] [CrossRef]
- Gómez, J.M.; González-Megías, A. Asymmetrical interactions between ungulates and phytophagous insects: Being different matters. Ecology 2002, 83, 203–211. [Google Scholar] [CrossRef]
- Allombert, S.; Stockton, S.A.; Martin, J.-L. A Natural Experiment on the Impact of Overabundant Deer on Forest Invertebrates. Conserv. Biol. 2005, 19, 1917–1929. [Google Scholar] [CrossRef]
- Martin, J.-L.; Stockton, S.A.; Allombert, S.; Gaston, A.J. Top-down and bottom-up consequences of unchecked ungulate browsing on plant and animal diversity in temperate forests: Lessons from a deer introduction. Biol. Invasions 2009, 12, 353–371. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Homage to Santa Rosalia or Why Are There So Many Kinds of Animals? Am. Nat. 2002, 93, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Southwood, T.R.E.; Brown, V.K.; Reader, P.M. The relationships of plant and insect diversities in succession. Biol. J. Linn. Soc. 1979, 12, 327–348. [Google Scholar] [CrossRef]
- Strong, D.R.; Lawton, J.H.; Southwood, R. Insects on Plants. Community Patterns and Mechanisms; Blackwell Scientific Publications: Oxford, UK, 1984. [Google Scholar]
- Halaj, J.; Ross, D.W.; Moldenke, A.R. Habitat structure and prey availability as predictors of the abundance and community organization of spiders in western Oregon forest canopies. J. Arachnol. 1998, 26, 203–220. [Google Scholar]
- Ziesche, T.M.; Roth, M. Influence of environmental parameters on small-scale distribution of soil-dwelling spiders in forests: What makes the difference, tree species or microhabitat? For. Ecol. Manag. 2008, 255, 738–752. [Google Scholar] [CrossRef]
- Bucher, R.; Entling, M.H. Contrasting effects of habitat fragmentation, population density, and prey availability on body condition of two orb-weaving spiders. Ecol. Ѐntomol. 2011, 36, 680–685. [Google Scholar] [CrossRef]
- Nyffeler, M. Prey selection of spiders in the field. J. Arachnol. 1999, 27, 317–324. [Google Scholar]
- Katagiri, N.; Hijii, N. Effects of sika deer browsing on the arthropod communities on understory vegetation in a thinned Japanese cypress plantation. J. For. Res. 2015, 20, 347–356. [Google Scholar] [CrossRef]
- Landsman, A.P.; Bowman, J.L. Discordant response of spider communities to forests disturbed by deer herbivory and changes in prey availability. Ecosphere 2017, 8, e01703. [Google Scholar] [CrossRef]
- Meier, M.; Stöhr, D.; Walde, J.; Tasser, E. Influence of ungulates on the vegetation composition and diversity of mixed deciduous and coniferous mountain forest in Austria. Eur. J. Wildl. Res. 2017, 63, 29. [Google Scholar] [CrossRef]
- Roberson, E.J.; Chips, M.J.; Carson, W.P.; Rooney, T.P. Deer herbivory reduces web-building spider abundance by simplifying forest vegetation structure. PeerJ 2016, 4, 2538. [Google Scholar] [CrossRef] [Green Version]
- Bundesamt für Naturschutz (BfN). Vegetationskarte: Karte der Potentiellen Natürlichen Vegetation Deutschlands (PNV). 2004. Available online: http://www.floraweb.de/vegetation/vegetationskarte.html (accessed on 8 November 2017).
- Hessisches Landesamt für Naturschutz, Umwelt und Geologie (HLNUG). Umweltatlas Hessen: Die Naturräume Hessens und ihre Haupteinheiten. 2013. Available online: http://atlas.umwelt.hessen.de/servlet/Frame/atlas/naturschutz/naturraum/texte/ngl-wb.htm (accessed on 8 November 2017).
- Gerst, M.; Sundermann, M.; Westphal, P. Nachhaltigkeitsbericht für 2017; Landesbetrieb HessenForst: Kassel, Germany, 2018. [Google Scholar]
- Kinser, A.; Koope, K.; Freiherr von Münchhausen, H. Die Rotwildverbreitung in Deutschland. AFZ-DerWald 2010, 5, 32–34. [Google Scholar]
- Brook, A.J.; Woodcock, B.A.; Sinka, M.; Vanbergen, A.J. Experimental verification of suction sampler capture efficiency in grasslands of differing vegetation height and structure. J. Appl. Ecol. 2008, 45, 1357–1363. [Google Scholar] [CrossRef]
- Stewart, A.J.A.; Wright, A.F. A new inexpensive suction apparatus for sampling arthropods in grassland. Ecol. Ѐntomol. 1995, 20, 98–102. [Google Scholar] [CrossRef]
- Cardoso, P.; Pekár, S.; Jocqué, R.; Coddington, J.A. Global Patterns of Guild Composition and Functional Diversity of Spiders. PLoS ONE 2011, 6, e21710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Linear Mixed-Effects Models Using ’Eigen’ and S4. Available online: https://cran.r-project.org/web/packages/lme4/lme4.pdf (accessed on 14 January 2018).
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems: Data exploration. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Harrison, X.A. Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2014, 2, e616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolker, B.M.; Brooks, M.E.; Clark, C.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 14 January 2018).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Perea, R.; Girardello, M.; Miguel, A.S. Big game or big loss? High deer densities are threatening woody plant diversity and vegetation dynamics. Biodivers. Conserv. 2014, 23, 1303–1318. [Google Scholar] [CrossRef]
- Rooney, T.P.; Waller, D.M. Direct and indirect effects of white-tailed deer in forest ecosystems. For. Ecol. Manag. 2003, 181, 165–176. [Google Scholar] [CrossRef]
- Finzi, A.C.; Canham, C.D. Sapling growth in response to light and nitrogen availability in a southern New England forest. For. Ecol. Manag. 2000, 131, 153–165. [Google Scholar] [CrossRef]
- Košulič, O.; Michalko, R.; Hula, V. Impact of canopy openness on spider communities: Implications for conservation man-agement of formerly coppiced oak forests. PLoS ONE 2016, 11, e0148585. [Google Scholar] [CrossRef] [Green Version]
- Rooney, T.P. High white-tailed deer densities benefit graminoids and contribute to biotic homogenization of forest ground-layer vegetation. Plant Ecol. 2009, 202, 103–111. [Google Scholar] [CrossRef]
- Terborgh, J.; Estes, J.A. Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature; Island Press: Washington, DC, USA, 2010. [Google Scholar]
- Rooney, T.P. Deer impacts on forest ecosystems: A North American perspective. For. An Int. J. For. Res. 2001, 74, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Den Herder, M.; Virtanen, R.; Roininen, H. Effects of reindeer browsing on tundra willow and its associated insect herbi-vores. J. Appl. Ecol. 2004, 41, 870–879. [Google Scholar] [CrossRef]
- Bressette, J.W.; Beck, H.; Beauchamp, V.B. Beyond the browse line: Complex cascade effects mediated by white-tailed deer. Oikos 2012, 121, 1749–1760. [Google Scholar] [CrossRef]
- Barton, P.S.; Cunningham, S.A.; Lindenmayer, D.B.; Manning, A.D. The role of carrion in maintaining biodiversity and ecological processes in terrestrial ecosystems. Oecologia 2013, 171, 761–772. [Google Scholar] [CrossRef]
- Jay-Robert, P.; Niogret, J.; Errouissi, F.; Labarussias, M.; Paoletti, É.; Luis, M.V.; Lumaret, J.-P. Relative efficiency of extensive grazing vs. wild ungulates management for dung beetle conservation in a heterogeneous landscape from Southern Europe (Scarabaeinae, Aphodiinae, Geotrupinae). Biol. Conserv. 2008, 141, 2879–2887. [Google Scholar] [CrossRef]
- Miyashita, T.; Takada, M.; Shimazaki, A. Indirect effects of herbivory by deer reduce abundance and species richness of web spiders. Écoscience 2004, 11, 74–79. [Google Scholar] [CrossRef]
- Takada, M.; Baba, Y.G.; Yanagi, Y.; Terada, S.; Miyashita, T. Contrasting Responses of Web-Building Spiders to Deer Browsing Among Habitats and Feeding Guilds. Environ. Ѐntomol. 2008, 37, 938–946. [Google Scholar] [CrossRef]
- Rypstra, A.L. The importance of food and space in limiting web-spider densities; a test using field enclosures. Oecologia 1983, 59, 312–316. [Google Scholar] [CrossRef]
- Bucher, R.; Nickel, H.; Kaib, S.; Will, M.; Carchi, J.; Farwig, N.; Schabo, D.G. Birds and plants as indicators of arthropod spe-cies richness in temperate farmland. Ecol. Indic. 2019, 103, 272–279. [Google Scholar] [CrossRef]
- Roberts, M.J. Collins Field Guide: Spiders of Britain and Northern Europe; HarperCollins Publishers Ltd.: London, UK, 1995. [Google Scholar]
- Schuldt, A.; Fahrenholz, N.; Brauns, M.; Migge-Kleian, S.; Platner, C.; Schaefer, M. Communities of ground-living spiders in deciduous forests: Does tree species diversity matter? Biodivers. Conserv. 2008, 17, 1267–1284. [Google Scholar] [CrossRef] [Green Version]
- Uetz, G.W. Habitat structure and spider foraging. In Habitat Structure: The Physical Arrangement of Objects in Space; Bell, S.S., McCoy, E.D., Mushinsky, H.R., Eds.; Chapman & Hall: London, UK, 1991; pp. 325–348. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucher, R.; Rochlitz, J.; Wegner, N.; Heiß, A.; Grebe, A.; Schabo, D.G.; Farwig, N. Deer Exclusion Changes Vegetation Structure and Hunting Guilds of Spiders, but Not Multitrophic Understory Biodiversity. Diversity 2021, 13, 25. https://doi.org/10.3390/d13010025
Bucher R, Rochlitz J, Wegner N, Heiß A, Grebe A, Schabo DG, Farwig N. Deer Exclusion Changes Vegetation Structure and Hunting Guilds of Spiders, but Not Multitrophic Understory Biodiversity. Diversity. 2021; 13(1):25. https://doi.org/10.3390/d13010025
Chicago/Turabian StyleBucher, Roman, Jonas Rochlitz, Nathalie Wegner, Anna Heiß, Alexander Grebe, Dana G. Schabo, and Nina Farwig. 2021. "Deer Exclusion Changes Vegetation Structure and Hunting Guilds of Spiders, but Not Multitrophic Understory Biodiversity" Diversity 13, no. 1: 25. https://doi.org/10.3390/d13010025
APA StyleBucher, R., Rochlitz, J., Wegner, N., Heiß, A., Grebe, A., Schabo, D. G., & Farwig, N. (2021). Deer Exclusion Changes Vegetation Structure and Hunting Guilds of Spiders, but Not Multitrophic Understory Biodiversity. Diversity, 13(1), 25. https://doi.org/10.3390/d13010025