Unequivocal Differences in Predation Pressure on Large Carabid Beetles between Forestry Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Three-Dimensional Printed Decoys and Field Experiment
2.3. Data Analyses
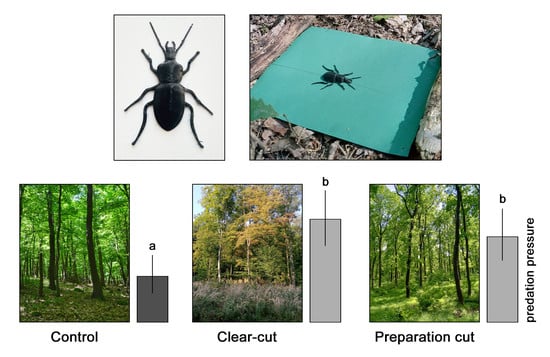
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lövei, G.L.; Sunderland, K.D. Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annu. Rev. Entomol. 1996, 41, 231–256. [Google Scholar] [CrossRef]
- Brandmayr, P.; Bonacci, T.; Giglio, A.; Talarico, F.F.; Brandmayr, T.Z. The evolution of defence mechanisms in carabid beetles: A review. In Life and Time: The Evolution of Life and Its History; Casellato, S., Burighel, P., Minelli, A., Eds.; Cleup: Padova, Italy, 2009; pp. 25–43. [Google Scholar]
- Sugiura, S. Predators as drivers of insect defenses. Entomol. Sci. 2020, 23, 316–337. [Google Scholar] [CrossRef]
- Giglio, A.; Vommaro, M.L.; Brandmayr, P.; Talarico, F. Pygidial Glands in Carabidae, an Overview of Morphology and Chemical Secretion. Life 2021, 11, 562. [Google Scholar] [CrossRef]
- Niehues, F.J.; Hockmann, P.; Weber, F. Genetics and dynamics of a Carabus auronitens metapopulation in the Westphalian Lowlands (Coleoptera, Carabidae). Ann. Zool. Fenn. 1996, 33, 85–96. [Google Scholar]
- Riecken, U.; Raths, U. Use of radio telemetry for studying dispersal and habitat use of Carabus coriaceus L. Ann. Zool. Fenn. 1996, 33, 109–116. [Google Scholar]
- Růžičková, J.; Veselý, M. Movement activity and habitat use of Carabus ullrichii (Coleoptera: Carabidae): The forest edge as a mating site? Entomol. Sci. 2018, 21, 76–83. [Google Scholar] [CrossRef]
- Elek, Z.; Růžičková, J.; Ódor, P. Individual decisions drive the changes in movement patterns of ground beetles between forestry management types. Biologia 2021, 1–10. [Google Scholar] [CrossRef]
- Ferrante, M.; Cacciato, A.L.; Lövei, G.L. Quantifying predation pressure along an urbanisation gradient in Denmark using artificial caterpillars. Eur. J. Entomol. 2014, 111, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Lövei, G.L.; Ferrante, M. A review of the sentinel prey method as a way of quantifying invertebrate predation under field conditions. Insect Sci. 2017, 24, 528–542. [Google Scholar] [CrossRef]
- Boetzl, F.A.; Konle, A.; Krauss, J. Aphid cards—Useful model for assessing predation rates or bias prone nonsense? J. Appl. Entomol. 2020, 144, 74–80. [Google Scholar] [CrossRef]
- Thiele, H.U. Carabid Beetles in Their Environments; Springer: Berlin/Heidelberg, Germany, 1997; 369p. [Google Scholar] [CrossRef]
- Graclik, A.; Wasielewski, O. Diet composition of Myotis myotis (Chiroptera, Vespertilionidae) in western Poland: Results of fecal analyses. Turk. J. Zool. 2012, 36, 209–213. [Google Scholar] [CrossRef]
- Fukuda, S.; Konuma, J. Using three-dimensional printed models to test for aposematism in a carabid beetle. Biol. J. Linn. Soc. Lond. 2019, 128, 735–741. [Google Scholar] [CrossRef]
- Negro, M.; Vacchiano, G.; Berretti, R.; Chamberlain, D.E.; Palestrini, C.; Motta, R.; Rolando, A. Effects of forest management on ground beetle diversity in alpine beech (Fagus sylvatica L.) stands. For. Ecol. Manag. 2014, 328, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Elek, Z.; Kovács, B.; Aszalós, R.; Boros, G.; Samu, F.; Tinya, F.; Ódor, P. Taxon-specific responses to different forestry treatments in a temperate forest. Sci. Rep. 2018, 8, 16990. [Google Scholar] [CrossRef] [Green Version]
- Elek, Z.; Růžičková, J.; Ódor, P. Functional plasticity of carabids can presume better the changes in community composition than taxon-based descriptors. Ecol. Appl. 2021, e02460. [Google Scholar] [CrossRef]
- Bengtsson, J.; Nilsson, S.G.; Franc, A.; Menozzi, P. Biodiversity, disturbances, ecosystem function and management of European forests. For. Ecol. Manag. 2000, 132, 39–50. [Google Scholar] [CrossRef]
- Ewers, R.M.; Didham, R.K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 2006, 81, 117–142. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Krauss, J.; Steffan-Dewenter, I. Predation rates on semi-natural grasslands depend on adjacent habitat type. Basic Appl. Ecol. 2013, 14, 614–621. [Google Scholar] [CrossRef]
- González-Gómez, P.L.; Estades, C.F.; Simonetti, J.A. Strengthened insectivory in a temperate fragmented forest. Oecologia 2006, 148, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Belovsky, G.E.; Slade, J.B.; Stockhoff, B.A. Susceptibility to predation for different grasshoppers: An experimental study. Ecology 1990, 71, 624–634. [Google Scholar] [CrossRef]
- Pitt, W.C. Effects of multiple vertebrate predators on grasshopper habitat selection: Trade-offs due to predation risk, foraging, and thermoregulation. Evol. Ecol. 1999, 13, 499–515. [Google Scholar] [CrossRef]
- Bartholomew, A.; El Moghrabi, J. Seasonal preference of darkling beetles (Tenebrionidae) for shrub vegetation due to high temperatures, not predation or food availability. J. Arid Environ. 2018, 156, 34–40. [Google Scholar] [CrossRef]
- Pearson, D.L. The function of multiple anti-predator mechanisms in adult tiger beetles (Coleoptera: Cicindelidae). Ecol. Entomol. 1985, 10, 65–72. [Google Scholar] [CrossRef]
- Sam, K.; Remmel, T.; Molleman, F. Material affects attack rates on dummy caterpillars in tropical forest where arthropod predators dominate: An experiment using clay and dough dummies with green colourants on various plant species. Entomol. Exp. Appl. 2015, 157, 317–324. [Google Scholar] [CrossRef]
- Howe, A.G.; Nachman, G.; Lövei, G.L. Predation pressure in Ugandan cotton fields measured by a sentinel prey method. Entomol. Exp. Appl. 2015, 154, 161–170. [Google Scholar] [CrossRef]
- Růžičková, J.; Bérces, S.; Ackov, S.; Elek, Z. Individual movement of large carabids as a link for activity density patterns in various forestry treatments. Acta Zool. Acad. Sci. Hung. 2021, 67, 77–86. [Google Scholar] [CrossRef]
- Tinya, F.; Kovács, B.; Prättälä, A.; Farkas, P.; Aszalós, R.; Ódor, P. Initial understory response to experimental silvicultural treatments in a temperate oak-dominated forest. Eur. J. For. Res. 2019, 138, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Kovács, B.; Tinya, F.; Guba, E.; Németh, C.; Sass, V.; Bidló, A.; Ódor, P. The short-term effects of experimental forestry treatments on site conditions in an oak–hornbeam forest. Forests 2018, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Kovács, B.; Tinya, F.; Németh, C.; Ódor, P. Unfolding the effects of different forestry treatments on microclimate in oak forests: Results of a 4-yr experiment. Ecol. Appl. 2020, 30, e02043. [Google Scholar] [CrossRef] [Green Version]
- Effect of Forestry Treatments on Forest Site, Regeneration and Biodiversity. An Experimental Study. Available online: https://piliskiserlet.ecolres.hu/en (accessed on 16 September 2021).
- Blender—A 3D Modelling and Rendering Package. Available online: http://www.blender.org (accessed on 16 September 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bartoń, K. MuMIn: Multi-Model Inference. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 16 September 2021).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information Theoretic Approach; Springer: New York, NY, USA, 2002; 488p. [Google Scholar]
- Low, P.A.; Sam, K.; McArthur, C.; Posa, M.R.C.; Hochuli, D.F. Determining predator identity from attack marks left in model caterpillars: Guidelines for best practice. Entomol. Exp. Appl. 2014, 152, 120–126. [Google Scholar] [CrossRef]
- Schley, L.; Roper, T.J. Diet of wild boar Sus scrofa in Western Europe, with particular reference to consumption of agricultural crops. Mammal Rev. 2003, 33, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, J.G. The Diet and Habitat Utilisation of the Badger (Meles meles) in an Area to the South of Durham City. Ph.D. Thesis, Durham University, Durham, UK, 1992. [Google Scholar]
- Marassi, M.; Biancardi, C. Diet of the Eurasian badger (Meles meles) in an area of the Italian Prealps. Hystrix It. J. Mamm. 2002, 13, 19–28. [Google Scholar] [CrossRef]
- Ries, L.; Fagan, W.F. Habitat edges as a potential ecological trap for an insect predator. Ecol. Entomol. 2003, 28, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.S. Effects of environmental stress on species rich assemblages. Biol. J. Linn. Soc. Lond. 1989, 37, 19–32. [Google Scholar] [CrossRef]
- Eötvös, C.B.; Lövei, G.L.; Magura, T. Predation pressure on sentinel insect prey along a riverside urbanization gradient in Hungary. Insects 2020, 11, 97. [Google Scholar] [CrossRef] [Green Version]
- Eötvös, C.B.; Magura, T.; Lövei, G.L. A meta-analysis indicates reduced predation pressure with increasing urbanization. Landsc. Urban Plan. 2018, 180, 54–59. [Google Scholar] [CrossRef]
- Niemelä, J.; Spence, J.R.; Spence, D.H. Habitat associations and seasonal activity of ground-beetles (Coleoptera, Carabidae) in central Alberta. Can. Entomol. 1992, 124, 521–540. [Google Scholar] [CrossRef]
- Pearce, J.L.; Venier, L.A.; McKee, J.; Pedlar, J.; McKenney, D. Influence of habitat and microhabitat on carabid (Coleoptera: Carabidae) assemblages in four stand types. Can. Entomol. 2003, 135, 337–357. [Google Scholar] [CrossRef]
- Wehnert, A.; Wagner, S. Niche partitioning in carabids: Single-tree admixtures matter. Insect Conserv. Divers. 2019, 12, 131–146. [Google Scholar] [CrossRef]
- Murray, B.D.; Holland, J.D.; Summerville, K.S.; Dunning, J.B.; Saunders, M.R.; Jenkins, M.A. Functional diversity response to hardwood forest management varies across taxa and spatial scales. Ecol. Appl. 2017, 27, 1064–1081. [Google Scholar] [CrossRef] [PubMed]
- Shochat, E.; Warren, P.S.; Faeth, S.H.; McIntyre, N.E.; Hope, D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 2006, 21, 186–191. [Google Scholar] [CrossRef] [PubMed]


| Cover of (Mean ± SEM, in %) | No. of Trees per Plot (Mean) | ||||
|---|---|---|---|---|---|
| Treatment | Bare Soil | Leaf Litter | Herbal Layer | Shrubs | |
| Control | 2.51 ± 0.52 | 68.70 ± 2.68 | 28.25 ± 2.59 | 0.58 ± 0.37 | 4.67 |
| Clear cut | 3.63 ± 1.04 | 10.91 ± 1.68 | 36.10 ± 2.20 | 49.35 ± 2.90 | 0.50 |
| Preparation cut | 4.11 ± 0.97 | 33.36 ± 2.39 | 50.40 ± 2.76 | 12.10 ± 2.14 | 2.67 |
| Model | df | LogLik | AICs | Delta | Weight |
|---|---|---|---|---|---|
| Spatio-temporal scale | 5 | −179.890 | 370.1 | 0.00 | 0.833 |
| Spatial scale | 3 | −183.925 | 374.0 | 3.86 | 0.121 |
| Micro-spatial scale | 6 | −181.705 | 375.9 | 5.77 | 0.046 |
| Model | Explanatory Variables | χ2 | df | p |
|---|---|---|---|---|
| Spatio-temporal scale | treatment | 11.334 | 2 | 0.003 |
| daytime | 4.444 | 1 | 0.035 | |
| season | 3.625 | 1 | 0.056 | |
| Spatial scale | treatment | 11.286 | 2 | 0.004 |
| Micro-spatial scale | treatment | 0.203 | 2 | 0.903 |
| leaf litter | 0.973 | 1 | 0.323 | |
| bare soil | 2.404 | 1 | 0.121 | |
| tree | 0.010 | 1 | 0.918 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Růžičková, J.; Elek, Z. Unequivocal Differences in Predation Pressure on Large Carabid Beetles between Forestry Treatments. Diversity 2021, 13, 484. https://doi.org/10.3390/d13100484
Růžičková J, Elek Z. Unequivocal Differences in Predation Pressure on Large Carabid Beetles between Forestry Treatments. Diversity. 2021; 13(10):484. https://doi.org/10.3390/d13100484
Chicago/Turabian StyleRůžičková, Jana, and Zoltán Elek. 2021. "Unequivocal Differences in Predation Pressure on Large Carabid Beetles between Forestry Treatments" Diversity 13, no. 10: 484. https://doi.org/10.3390/d13100484
APA StyleRůžičková, J., & Elek, Z. (2021). Unequivocal Differences in Predation Pressure on Large Carabid Beetles between Forestry Treatments. Diversity, 13(10), 484. https://doi.org/10.3390/d13100484