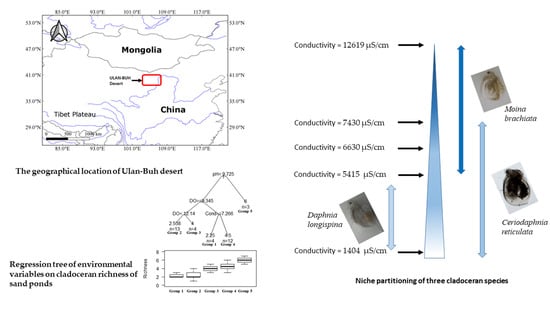
Species Diversity and Community Assembly of Cladocera in the Sand Ponds of the Ulan Buh Desert, Inner Mongolia of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Object
2.2. Field Sampling and Laboratory Work
2.3. Statistical Analysis
3. Results
3.1. Environmental Variables and Spatial Landscape
3.2. Species Diversity
3.3. Species Association and Coexistence
3.4. Variation of Cladoceran Communities along Environmental Gradients
3.5. Variation of the Cladoceran Community along Spatial Gradients
4. Discussion
4.1. Cladocera Diversity
4.2. Community Assembly
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Family | Species | Codes | Lake | Pond |
|---|---|---|---|---|
| Sididae Bard, 1850 | Diaphanosoma mongolianum Uéno, 1938 | dia | + | + |
| Daphniidae (Straus, 1820) | Daphnia magna Straus, 1820 | dam | + | + |
| Daphnia longispina (O. F. Müller, 1776) | dal | + | + | |
| Simocephalus exspinosus (De Geer, 1778) | sie | + | + | |
| Simocephalus vetulus (O. F. Müller, 1776) | siv | + | + | |
| Scapholeberis smirnovi Garibian et al., 2020 | sck | + | + | |
| Ceriodaphnia reticulata (Jurine, 1820) | cer | + | + | |
| Moinidae Goulden, 1968 | Moina cf brachiata (Leydig, 1860) | mor | + | + |
| Macrothricidae Norman & Brady, 1867 | Macrothrix rosea (Jurine, 1820) | mar | + | - |
| Macrothrix spinosa King, 1853 | mas | - | + | |
| Chydoridae Stebbing, 1902 | Oxyurella tenuicaudis (Sars, 1862) | oxt | - | + |
| Alona guttata Sars, 1862 | alg | + | + | |
| Coronatella rectangula (Sars, 1862) | cor | + | + | |
| Alonella nana (Baird, 1843) | aln | + | - | |
| Pleuroxus aduncus (Jurine, 1820) | pla | - | + | |
| Chydorus sphaericus (O. F. Müller, 1776) | chs | + | + |
References
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework from multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Figuerola, J.; Green, A.J. Dispersal of aquatic organisms by waterbirds: A review of past research and priorities for future studies. Freshw. Biol. 2002, 47, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Sirianni, K.M. Differential wind dispersal of cladoceran ephippia in a rock pool metacommunity. Aquat. Ecol. 2017, 51, 1–16. [Google Scholar] [CrossRef]
- Louette, G.; De Meester, L. High dispersal capacity of cladoceran zooplankton in newly founded communities. Ecology 2005, 86, 353–359. [Google Scholar] [CrossRef]
- Dodson, S.I.; Newman, A.L.; Willwolf, S.; Alexander, M.L.; Woodford, M.P.; Egeren, S.V. The relationship between zooplankton community structure and lake characteristics in temperate lakes (Northern Wisconsin, USA). J. Plankton Res. 2009, 31, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Declerck, S.A.J.; Coronel, J.S.; Legendre, P.; Brendonck, L. Scale dependency of processes structuring metacommunities of cladocerans in temporary pools of High-Andes wetlands. Ecography 2011, 34, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Walseng, B.; Yan, N.D.; Schartau, A.K. Littoral microcrustacean (Cladocera and Copepoda) indicators of acidification in Canadian Shield lakes. AMBIO 2003, 32, 208–213. [Google Scholar] [CrossRef]
- Chen, G.; Dalton, C.; Taylor, D. Cladocera as indicators of trophic state in Irish ponds. J. Paleolimnol. 2010, 44, 465–481. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Canterbury, G.E.; Martin, T.E.; Petit, D.R.; Petit, L.J.; Bradford, D.F. Bird Communities and Habitat as Ecological Indicators of Forest Condition in Regional Monitoring. Conserv. Biol. 2000, 14, 544–558. [Google Scholar] [CrossRef]
- Valero, E.; Álvarez, X.; Picos, J. An assessment of river habitat quality as an indicator of conservation status. A case study in the Northwest of Spain. Ecol. Indic. 2015, 57, 131–138. [Google Scholar] [CrossRef]
- Bakker, J.D. Increasing the utility of Indicator Species Analysis. J. Appl. Ecol. 2008, 45, 1829–1835. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, M.D.; Legendre, P.; Moretti, M. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P.; Wiser, S.K.; Brotons, L. Using species combinations in indicator value analyses. Methods Ecol. Evol. 2012, 3, 973–982. [Google Scholar] [CrossRef]
- Urban, N.A.; Swihart, R.K.; Malloy, M.C.; Dunning, J.B., Jr. Improving selection of indicator species when detection is imperfect. Ecol. Indic. 2011, 15, 188–197. [Google Scholar] [CrossRef]
- He, Z.; An, S. The adaptation to salinity in Moina brachiata. Chin. J. Zool. 1986, 2, 27–30. (In Chinese) [Google Scholar]
- Guo, F.F.; Dumont, H.J. Relict populations of Diaphanosoma (Cladocera: Ctenopoda) in the Chadian Sahara, with the description of a new species. Zootaxa 2014, 3856, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Kotov, A.A. Jurassic Cladocera (Crustacea, Branchiopoda) with a description of an extinct Mesozoic Order. J. Nat. Hist. 2007, 41, 13–37. [Google Scholar] [CrossRef]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. Chem. Methods 1996, 142, 31–33. [Google Scholar]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees. Wadsworth 1984, 20, 582–588. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Science Ltd.: Victoria, UK, 2004; pp. 1–215. [Google Scholar]
- Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 1987, 43, 783. [Google Scholar] [CrossRef] [PubMed]
- Dumont, H.J.; Segers, H. Estimating lacustrine zooplankton species richness and complementarity. Hydrobiologia 1996, 341, 125–132. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol. Model. 2002, 153, 51–68. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P.; Avoisjacquet, C.; Tuomisto, H. Dissecting the spatial structure of ecological data at multiple scales. Ecology 2004, 85, 1826–1832. [Google Scholar] [CrossRef] [Green Version]
- Chambers, J.M. SoDA: Functions and Examples for “Software for Data Analysis”. R package version 1.0-6.1. 2020. Available online: https://CRAN.R-project.org/package=SoDA (accessed on 28 October 2020).
- Chao, A.; Ma, K.H.; Hsieh, T.C.; Chiu, C.H. SpadeR: Species-Richness Prediction and Diversity Estimation with R. R package version 0.1.1. 2016. Available online: https://CRAN.R-project.org/package=SpadeR (accessed on 6 September 2016).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. R package version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 28 November 2020).
- Therneau, T.; Atkinson, B. rpart: Recursive Partitioning and Regression Trees. R package version 4.1-15. 2019. Available online: https://CRAN.R-project.org/package=rpart (accessed on 12 April 2019).
- Drenner, S.M.; Dodson, S.I.; Drenner, R.W.; Pinder, J.E. Crustacean zooplankton community structure in temporary and permanent grassland ponds. Hydrobiologia 2009, 632, 225–233. [Google Scholar] [CrossRef]
- Maiphae, S.; Pholpunthin, P.; Dumont, H.J. Species richness of the Cladocera (Branchiopoda: Anomopoda and Ctenopoda) in southern Thailand, and its complementarity with neighboring regions. Hydrobiologia 2005, 537, 147–156. [Google Scholar] [CrossRef]
- Dodson, S. Predicting crustacean zooplankton species richness. Limnol. Oceanogr. 1992, 37, 848–856. [Google Scholar] [CrossRef]
- Green, J.D. Plankton of lake Ototoa, a sand-dune lake in Northern New Zealand. N. Z. J. Mar. Fresh. 1976, 10, 43–59. [Google Scholar] [CrossRef]
- Chertoprud, E.S.; Sinev, A.Y.; Dimante-Deimantovica, I. Fauna of Cladocera and copepoda from Xinjiang Uyghur autonomous region (China). Zootaxa 2017, 4258, 561–573. [Google Scholar] [CrossRef]
- Van Damme, K.; Dumont, H.J. Cladocera of the Lençóis Maranhenses (NE-Brazil): Faunal composition and a reappraisal of Sars’ Method. Braz. J. Biol. 2010, 70, 755–779. [Google Scholar] [CrossRef] [PubMed]
- Afonina, E.Y.; Tashlykova, N.A. Plankton community and the relationship with the environment in saline lakes of Onon-Torey plain, Northeastern Mongolia. Saudi. J. Biol. Sci. 2018, 25, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Afonina, E.Y.; Tashlykova, N.A. Plankton of Saline Lakes in Southeastern Transbaikalia: Transformation and Environmental Factors. Contemp. Probl. Ecol. 2019, 12, 155–170. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Hu, L.; Leppänen, J.J. Cladoceran communities in soda lakes of the Badain Jaran desert, NW China. J. Arid Environ. 2020, 177, 104133. [Google Scholar] [CrossRef]
- Hu, L.; Li, Y.; Leppänen, J.J.; Chen, G.; Lang, W.; Wang, X.; Qiang, M. Human impacts on the cladoceran community of Jili Lake, arid NW China, over the past century. J. Paleolimnol. 2021, 1–12. [Google Scholar]
- Hanski, I.; Ranta, E. Coexistence in a Patchy Environment: Three Species of Daphnia in Rock Pools. J. Anim. Ecol. 1983, 52, 263–279. [Google Scholar] [CrossRef]
- De Meester, L.; Gómez, A.; Okamura, B.; Schwenk, K. The Monopolization Hypothesis and the dispersal–gene flow paradox in aquatic organisms. Acta Oecol. 2002, 23, 121–135. [Google Scholar] [CrossRef]
- Korovchinsky, N.M. Sididae & Holopediidae (Crustacea: Daphniiformes). In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 3; SPB Academic: Hague, The Netherlands, 1992; pp. 1–82. [Google Scholar]
- Horváth, Z.; Vad, C.F.; Tóth, A.; Zsuga, K.; Boros, E.; Vörös, L.; Ptacnik, R. Opposing patterns of zooplankton diversity and functioning along a natural stress gradient: When the going gets tough, the tough get going. Oikos 2014, 123, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Yılmaz, G.; Çolak, M.A.; Özgencil, İ.K.; Metin, M.; Korkmaz, M.; Ertuğrul, S.; Soyluer, M.; Bucak, T.; Tavşanoğlu, Ü.N.; Özkan, K.; et al. Decadal changes in size, salinity, waterbirds, and fish in lakes of the Konya Closed Basin, Turkey, associated with climate change and increasing water abstraction for agriculture. Inland Waters 2021, 1–18. [Google Scholar] [CrossRef]
- Lin, Q.Q.; Xu, L.; Hou, J.Z.; Liu, Z.W.; Jeppesen, E.; Han, B.P. Responses of trophic structure and zooplankton community to salinity and temperature in Tibetan lakes: Implication for the effect of climate warming. Water Res. 2017, 124, 618–629. [Google Scholar] [CrossRef]




| Western | Eastern | p Value | |
|---|---|---|---|
| Altitude | 1032.5 | 1044.86 | p < 0.001 |
| Pond size (m2) | 151.43 | 311.36 | p = 0.492 |
| Depth (m) | 0.87 | 1.1 | p = 0.203 |
| Conductivity(mS/cm) | 6.15 | 3.03 | p < 0.001 |
| DO (mg/L) | 9.12 | 10.17 | p = 0.238 |
| pH | 9.06 | 9.22 | p = 0.322 |
| Vegetation coverage | 0.66 | 0.75 | p = 0.233 |
| Average richness | 3.43 | 3.73 | p = 0.557 |
| Total species richness | 13 | 11 | |
| Restricted species | Diaphanosoma mongolianum Moina cf brachiata Pleuroxus aduncus | Macrothrix spinosa | |
| Estimate | SE | 95% Lower | 95% Upper | |
|---|---|---|---|---|
| Homogeneous Model | 12.767 | 1.063 | 14.101 | 19.838 |
| Chao2 (Chao, 1987) | 18.375 | 7.003 | 14.480 | 53.842 |
| Chao2-bc | 15.458 | 2.534 | 14.145 | 28.716 |
| iChao2 (Chiu et al., 2014) | 18.375 | 5.940 | 14.590 | 46.445 |
| ICE (Lee & Chao, 1994) | 15.954 | 2.437 | 14.293 | 27.046 |
| ICE-1 (Lee & Chao, 1994) | 16.441 | 3.274 | 14.334 | 31.826 |
| 1st order jackknife | 16.917 | 2.398 | 14.713 | 25.930 |
| 2nd order jackknife | 18.833 | 4.086 | 15.146 | 34.385 |
| Indicator Species | Associated Environment | Specificity | Fidelity | p-Value |
|---|---|---|---|---|
| mor | Conductivty: 6630–12619 μS/cm | 0.947 | 0.667 | 0.005 ** |
| cor + mor | Conductivty: 6630–12619 μS/cm | 0.933 | 0.556 | 0.004 ** |
| cer + mor | Conductivty: 6630–7430 μS/cm | 0.857 | 0.500 | 0.040 * |
| cer | Conductivty: 1404–7430 μS/cm | 1.000 | 0.939 | 0.001 *** |
| cor + cer | Conductivty: 1404–7430 μS/cm | 1.000 | 0.818 | 0.025 * |
| dal | Conductivty: 1404–5415 μS/cm | 1.000 | 0.519 | 0.05 * |
| chs + sck | pH:9.82–10.06 | 0.909 | 1.000 | 0.001 *** |
| chs | pH:8.92–8.94 & 9.82–10.06 | 0.811 | 0.800 | 0.012 * |
| cor + chs | pH:8.92–8.94 & 9.82–10.06 | 0.811 | 0.800 | 0.012 * |
| cer + chs | pH:8.92–8.94 & 9.82–10.06 | 0.811 | 0.800 | 0.012 * |
| cor + sck | pH:8.36–8.5 & 9.13–9.19 & 9.82–10.06 | 0.769 | 0.778 | 0.028 * |
| cor + mor | Tadpole present | 0.875 | 0.333 | 0.040 * |
| cor + dal | Tadpole absent | 0.797 | 0.524 | 0.033 * |
| cor + mor | Fish present | 0.895 | 1.000 | 0.025 * |
| mor | Fish present | 0.872 | 1.000 | 0.036 * |
| mor | Vegetation coverage: 50% | 0.706 | 1.000 | 0.049 * |
| Df | R | Radj | Testable | |
|---|---|---|---|---|
| Presence/Absence | ||||
| E | 2 | 17.4% | 12.4% | TRUE |
| S1 | 2 | 13.9% | 8.7% | TRUE |
| E + S1 | 4 | 26.5% | 17.0% | TRUE |
| Shared | 0 | 4.1% | FALSE | |
| E|S1 | 2 | 8.3% | TRUE | |
| S1|E | 2 | 4.6% | TRUE | |
| Residuals | 83.0% | FALSE | ||
| Relative abundance | ||||
| E | 2 | 30.0% | 25.8% | TRUE |
| S2 | 3 | 23.2% | 15.9% | TRUE |
| E + S2 | 5 | 45.6% | 36.5% | TRUE |
| shared | 0 | 5.2% | FALSE | |
| E|S2 | 2 | 20.6% | TRUE | |
| S2|E | 3 | 10.7% | TRUE | |
| Residuals | 63.5% | FALSE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.-L.; Huang, Q.; Xu, L.; Rizo, E.Z.; Alonso, M.; Dumont, H.J.; Han, B.-P. Species Diversity and Community Assembly of Cladocera in the Sand Ponds of the Ulan Buh Desert, Inner Mongolia of China. Diversity 2021, 13, 502. https://doi.org/10.3390/d13100502
Gu Y-L, Huang Q, Xu L, Rizo EZ, Alonso M, Dumont HJ, Han B-P. Species Diversity and Community Assembly of Cladocera in the Sand Ponds of the Ulan Buh Desert, Inner Mongolia of China. Diversity. 2021; 13(10):502. https://doi.org/10.3390/d13100502
Chicago/Turabian StyleGu, Yang-Liang, Qi Huang, Lei Xu, Eric Zeus Rizo, Miguel Alonso, Henri J. Dumont, and Bo-Ping Han. 2021. "Species Diversity and Community Assembly of Cladocera in the Sand Ponds of the Ulan Buh Desert, Inner Mongolia of China" Diversity 13, no. 10: 502. https://doi.org/10.3390/d13100502
APA StyleGu, Y. -L., Huang, Q., Xu, L., Rizo, E. Z., Alonso, M., Dumont, H. J., & Han, B. -P. (2021). Species Diversity and Community Assembly of Cladocera in the Sand Ponds of the Ulan Buh Desert, Inner Mongolia of China. Diversity, 13(10), 502. https://doi.org/10.3390/d13100502