Temporal and Spatial Variations of Geodia cydonium (Jameson) (Porifera, Demospongiae) in the Mediterranean Confined Environments
Abstract
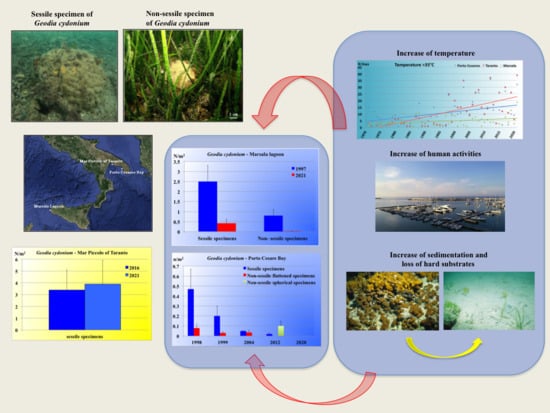
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.1.1. Marsala Lagoon
2.1.2. Mar Piccolo of Taranto
2.1.3. Porto Cesareo Bay
2.2. Survey Methods
2.3. Statistical Analysis
3. Results
3.1. Marsala Lagoon
3.2. Mar Piccolo of Taranto
3.3. Porto Cesareo Bay
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steffen, W. Trajectories of the earth system in the Antropocene. Proc. Natl. Acad. Sci. USA 2018, 115, 8252–8259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trisos, C.H.; Merow, C.; Pigot, A.L. The projectd timing of abrupt ecological disruption from climate change. Nature 2020, 580, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Albano, P.G.; Steger, J.; Bošnjak, M.; Dunne, B.; Guifarro, Z.; Turapova, E.; Hua, Q.; Kaufman, D.S.; Rilov, G.; Zuschin, M. Native biodiversity collapse in the eastern Mediterranean. Proc. R. Soc. B Boil. Sci. 2021, 288, 20202469. [Google Scholar] [CrossRef] [PubMed]
- Torresan, S.; Gallina, V.; Gualdi, S.; Bellafiore, D.; Umgiesser, G.; Carniel, S.; Sclavo, M.; Benetazzo, A.; Giubilato, E.; Critto, A. Assessment of Climate Change Impacts in the North Adriatic Coastal Area. Part I: A Multi-Model Chain for the Definition of Climate Change Hazard Scenarios. Water 2019, 11, 1157. [Google Scholar] [CrossRef] [Green Version]
- Marzano, C.N.; Baldacconi, R.; Fianchini, A.; Gravina, F.; Corriero, G. Settlement seasonality and temporal changes in hard substrate macrozoobenthic communities of Lesina Lagoon (Apulia, Southern Adriatic Sea). Chem. Ecol. 2007, 23, 479–491. [Google Scholar] [CrossRef]
- Cardone, F.; Corriero, G.; Fianchini, A.; Gravina, M.F.; Marzano, C.N. Biodiversity of transitional waters: Species composition and comparative analysis of hard bottom communities from the south-eastern Italian coast. J. Mar. Biol. Assoc. UK 2014, 94, 25–34. [Google Scholar] [CrossRef]
- Gristina, M.; Cardone, F.; Carlucci, R.; Castellano, L.; Passarelli, S.; Corriero, G. Abundance, distribution and habitat preference of Hippocampus guttulatus and Hippocampus hippocampus in a semi-enclosed central Mediterranean marine area. Mar. Ecol. 2015, 36, 57–66. [Google Scholar] [CrossRef]
- Piscitelli, M.; Corriero, G.; Gaino, E.; Uriz, M.-J. Reproductive cycles of the sympatric excavating sponges Cliona celata and Cliona viridis in the Mediterranean Sea. Invertebr. Biol. 2011, 130, 1–10. [Google Scholar] [CrossRef]
- Hirose, Y.; Aini, S.N.; Yamashiro, H. Contact reactions between individuals of the coral-killing sponge, Terpios hoshinota. Zool. Stud. 2021, 60, 41. [Google Scholar]
- Longo, C.; Cardone, F.; Mercurio, M.; Marzano, C.N.; Pierri, C.; Corriero, G. Spatial and temporal distributions of the sponge fauna insouthern Italian lagoon systems. Mediterr. Mar. Sci. 2015, 17, 174. [Google Scholar] [CrossRef] [Green Version]
- Corriero, G. The sponge fauna from the Stagnone di Marsala (Sicily): Taxonomic and ecological observations. Boll. Mus. Ist. Biol. Univ. Genova 1989, 53, 101–113. [Google Scholar]
- Corriero, G.; Longo, C.; Mercurio, M.; Marchini, A.; Occhipinti-Ambrogi, A. Benthic taxocoenoses on artificial hard-bottoms in the Venice Lagoon: Spatial distribution and temporal changes in the northern basin. Ital. J. Zool. 2007, 74, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Longo, C.; Scalera Liaci, L.; Corriero, G. Note sui poriferi del Mar Grande e del Mar Piccolo di Taranto (Mar Ionio). Biol. Mar. Medit. 2004, 11, 440–443. [Google Scholar]
- Corriero, G. Distribuzione ed Ecologia dei Poriferi in Ambienti Cnfinati Mediterranei. Ph.D. Thesis, University of Genoa, Genova, Italy, 1990. [Google Scholar]
- Scalera Liaci, L.; Sciscioli, M.; Fiordiponti, F. Distribuzione dei Poriferi del Mar Piccolo di Taranto. Oebalia 1976, 2, 3–19. [Google Scholar]
- Pulitzer-Finali, G. A collection of Mediterranean Demospongiae (Porifera) with, in appendix, a list of the Demospongiae hitherto recorded from the Mediterranean Sea. Ann. Mus. Civ. Stor. Nat. Giacomo Doria 1983, 84, 445–621. [Google Scholar]
- Mercurio, M. Distribuzione, Ecologia e Sperimentazione di Tecniche di Restocking della Fauna a Poriferi Presso L’insenatura della Strea di Porto Cesareo. Ph.D. Thesis, University of Bari, Bari, Italy, 2000. [Google Scholar]
- Mercurio, M.; Longo, C.; Nonnis Marzano, C.; Scalera Liaci, L.; Corriero, G. Demosponge di ambienti lagunari mediterranei. Biol. Mar. Medit. 2004, 11, 444–447. [Google Scholar]
- Mercurio, M.; Corriero, G.; Gaino, E. Sessile and non-sessile morphs of Geodia cydonium (Jameson) (Porifera, Demospongiae) in two semi-enclosed Mediterranean bays. Mar. Biol. 2006, 148, 489–501. [Google Scholar] [CrossRef]
- Uriz, M.J. Estudio sistematico de las esponjas Astrophorida (Demospongia) de los fondos de pesca de arrastre, entre Tossa y Calella (Cataluña). Bol. Inst. Espa. Oceano 1981, 6, 8–58. [Google Scholar]
- Morri, C.; Cinelli, F.; Bianchi, C.N. Sessile epifauna gigantismin a submarine cave with sulphur springs. Cave Diving 1994, 6, 4–9. [Google Scholar]
- Bertolino, M.; Cerrano, C.; Bavestrello, G.; Carella, M.; Pansini, M.; Calcina, B. Diversity of Porifera in the Mediterranean coralli-genous accretions, with description of a new species. Zookeys 2013, 336, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Santucci, R. La Geodia cydonium come centro di associazione biologica. Mem. CIII Com. Talas It. 1022, 103, 5–19. Available online: http://www.marinespecies.org/aphia.php?p=sourcedetails&id=354698 (accessed on 5 May 2020).
- Müller, W.E.; Wimmer, W.; Schatton, W.; Böhm, M.; Batel, R.; Filic, Z. Initiation of an Aquaculture of Sponges for the Sustainable Production of Bioactive Metabolites in Open Systems: Example, Geodia cydonium. Mar. Biotechnol. 1999, 1, 569–579. [Google Scholar] [CrossRef]
- Riggio, S.; Sparla, M.P. Notes on fauna inhabiting Rytyphlöea tinctoria (Clem) C Ag aegagropyla in the stagnone Sound (Western Sicily). Rapp. Comm. Int. Mer. Medit. 1985, 29, 143–144. [Google Scholar]
- Gherardi, M.; Giangrande, A.; Corriero, G. Epibiontic and endobiontic polychaetes of Geodia cydonium (Porifera, Demospongiae) from the Mediterranean Sea. Hydrobiology 2001, 443, 87–101. [Google Scholar] [CrossRef]
- Magazzù, G. Usefulness of the Marsala lagoon for acquaculture. Nutrients and primary production. Rapp. P-v Réun. Comm. Int. Explor. Scient. Mer. Méditerr. 1977, 24, 81–82. [Google Scholar]
- Basset, A.; Galuppo, N.; Sabetta, L. Environmental heterogeneity and benthic macroinvertebrate guilds in Italian lagoons. Transit. Waters Bull. 2006, 1, 48–63. [Google Scholar]
- Corriero, G. Note sui popolamenti di poriferi dello Stagnone di Marsala (Sicilia). Nova Thalass. 1984, 6, 213–223. [Google Scholar]
- Mercurio, M.; Corriero, G.; Scalera Liaci, L. Sulla forma non sessile di Geodia cydonium (Jameson) in un ambiente superficiale. Biol. Mar. Medit. 1997, 4, 407–409. [Google Scholar]
- Scardi, M.; Vinci, D.; Lanera, P.; Casolaro, R.; Valiante, L.M.; Plastina, N.; Di Dato, P. Studio ambientale per il recupero produttivo del Mar Piccolo di Taranto. AGCI-ICRMARE 1997, 6–74. [Google Scholar]
- Capasso, V.; Di Liddo, A.; Notarnicola, F.; Posa, D.; Rinaldi, F. Modelli matematici per il controllo della qualità delle acque costiere. In Proceedings of the Convegno Nazionale Progetto Strategico CNR ‘Matematica computazionale’, Rome, Italy, 2–4 May 1989. [Google Scholar]
- Gaino, E.; Cardone, F.; Corriero, G. Reproduction of the intertidal sponge Hymeniacidon perlevis (Montagu) along a bathymetric gradient. Open Mar. Biol. J. 2010, 4, 47–56. [Google Scholar] [CrossRef]
- Tursi, A.; Pastore, M.; Panetta, P. Aspetti ecologici del Mar Piccolo di Taranto: Ascidie, Crostacei Decapodi e Molluschi. In Proceedings of the IV Simposio Nazionale sulla Conservazione della Natura, Bari, Italy, 23–28 April 1974; pp. 93–117. [Google Scholar]
- Mastrototaro, F.; D’Onghia, G.; Tursi, A. Spatial and seasonal distribution of ascidians in a semi-enclosed basin of the Mediterranean Sea. J. Mar. Biol. Assoc. UK 2008, 88, 1053–1061. [Google Scholar] [CrossRef]
- Passeri, L. Sedimentazione carbonatica attuale e diagenesi precoce nella laguna di Porto Cesareo (Penisola Salentina). Boll. Soc. Geol. Ital. 1974, 92, 3–40. [Google Scholar]
- Corriero, G.; Scalera Liaci, L.; Mercurio, M. Il popolamento a Poriferi della Riserva Marina di Porto Cesareo. In Proceedings of the 57th National Congress UZI, San Benetto del Tronto, Italy, 22–26 September 1996; p. 28. [Google Scholar]
- Mercurio, M.; Scalera Liaci, L.; Corriero, G. La fauna a poriferi del bacino della Strea di Porto Cesareo (LE). Biol. Mar. Medit. 2001, 8, 403–412. [Google Scholar]
- Parenzan, P. Un habitat marino di tipo subtropicale a Porto Cesareo. In Proceedings of the VI Simposio Nazionale sulla Conservazione della Natura, Bari, Italy, 26–29 April 1976; pp. 151–157. [Google Scholar]
- Mercurio, M.; Nonnis Marzano, C.; Scalera Liaci, L.; Corriero, G. L’insenatura della Strea di Porto Cesareo (LE): Da sponge gardend a sand bed. In Proceedings of the 65th National Congress UZI, Taormina, Italy, 21–25 September 2004. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Ponti, M.; Fava, F.; Abbiati, M. Spatial–temporal variability of epibenthic assemblages on subtidal biogenic reefs in the northern Adriatic Sea. Mar. Biol. 2011, 158, 1447–1459. [Google Scholar] [CrossRef]
- Turicchia, E.; Poli, D.; Abbiati, M.; Ponti, M. Abundance, size, and growth rate of Geodia cydonium (Demospongiae: Geodiidae) in the northern Adriatic temperate biogenic reefs. In Proceedings of the 40eme Congrès de la Commission Internationale pour l’Exploration Scientifique de la mer Méditerranée. CIESM, Marseille, France, 28 October 2013. [Google Scholar]
- Bastari, A.; Beccacece, J.; Ferretti, F.; Micheli, F.; Cerrano, C. Local Ecological Knowledge Indicates Temporal Trends of Benthic Invertebrates Species of the Adriatic Sea. Front. Mar. Sci. 2017, 4, 157. [Google Scholar] [CrossRef]
- Ferrer Hernández, F. Esponjas del Cantábrico. Parte III. Myxospongida. IV. Tetraxonida. V. Triaxonida. Trabajos del Museo Nacional de Ciencias Naturales (Zoológica) 1914, 17, 1–46. [Google Scholar]
- Topsent, E. Spongiaires de l’Atlantique et de la Méditerranée Provenant des Croisières du Prince Albert ler de Monaco. In Résultats des Campagnes Scientifiques Accomplies par le Prince Albert I. Monac. 1928. Available online: https://www.worldcat.org/title/spongiaires-de-latlantique-et-de-la-mediterranee-provenant-des-croisieres-du-prince-albert-ier-de-monaco/oclc/458332158 (accessed on 22 November 2021).
- Voultsiadou, E. Sponge diversity in the Aegean Sea: Check list and new information. Ital. J. Zoöl. 2005, 72, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Stjepčević, J.; Parenzan, P. Il Golfo delle Bocche di Cattaro—Condizioni generali e biocenosi bentoniche con carta ecologica delle sue due baie interne. Stud. Mar. 1980, 9, 3–149. [Google Scholar]
- Costa, G.; Bavestrello, G.; Micaroni, V.; Pansini, M.; Strano, F.; Bertolino, M. Sponge community variation along the Apulian coasts (Otranto Strait) over a pluri-decennial time span. Does water warming drive a sponge diversity increasing in the Mediterranean Sea? J. Mar. Biol. Assoc. UK 2019, 99, 1519–1534. [Google Scholar] [CrossRef]
- Grech, D.; Van De Poll, B.; Bertolino, M.; Rosso, A.; Guala, I. Massive stranding event revealed the occurrence of an overlooked and ecosystem engineer sponge. Mar. Biodivers. 2020, 50, 1–12. [Google Scholar] [CrossRef]
- Ponti, M.; Turicchia, E.; Rossi, G.; Cerrano, C. Reef Check Med—Key Mediterranean Marine Species 2001–2020; Reef Check Italia Onlus. Available online: https://doi.org/10.14284/468 (accessed on 5 May 2020).
- Genchi, C.; Calvo, S.; Lugaro, A.; Ragonese, S. Idrologia di una laguna costiera e caratterizzazione chimico-fisica dei sedimenti recenti in relazione alla distribuzione dei popolamenti vegetali sommersi (lo Stagnone di Marsala). Quad. IRPEM 1983, 4, 23–34. [Google Scholar]
- Cinelli, F.; Cognetti, G.; Grasso, M.; Mongelli, S.; Orlando, E.; Pagliai, A.M. Studio Ecologico dell’area Marina di Porto Cesareo; Congedo, Ed.; Studio Ecologico dell’area marina di Porto Cesareo: Lecce, Italy, 1988; pp. 1–388. [Google Scholar]
- Gravina, M.F.; Bonifazi, A.; Del Pasqua, M.; Giampaoletti, J.; Lezzi, M.; Ventura, D.; Giangrande, A. Perception of Changes in Marine Benthic Habitats: The Relevance of Taxonomic and Ecological Memory. Diversity 2020, 12, 480. [Google Scholar] [CrossRef]
- Calvo, S.; Ragonese, S. Osservazioni su Rytiphloea tinctoria (Clem.) C. Ag. in forma aegagropila nelle acque dello Stagnone (costa occidentale della Sicilia). Plant Biosyst. 1982, 116, 81–87. [Google Scholar] [CrossRef]
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Gristina, M.; Knittweis, L.; Martin, C.S.; Pergent, G. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, srep12505. [Google Scholar] [CrossRef] [Green Version]
- Calvo, S.; Calvo, R.; Luzzu, F.; Raimondi, V.; Assenzo, M.; Cassetti, F.; Tomasello, A. Performance Assessment of Posidonia oceanica (L.) Delile Restoration Experiment on Dead matte Twelve Years after Planting—Structural and Functional Meadow Features. Water 2021, 13, 724. [Google Scholar] [CrossRef]
- Rivetti, I.; Fraschetti, S.; Lionello, P.; Zambianchi, E.; Boero, F. Global Warming and Mass Mortalities of Benthic Invertebrates in the Mediterranean Sea. PLoS ONE 2014, 9, e115655. [Google Scholar] [CrossRef] [Green Version]
- Garrabou, J.; Coma, R.; Bensoussan, N.; Bally, M.; Chevaldonné, P.; Cigliano, M.; Diaz, D.; Harmelin, J.G.; Gambi, M.C.; Kersting, D.K.; et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Glob. Chang. Biol. 2009, 15, 1090–1103. [Google Scholar] [CrossRef]
- Hirose, E.; Nozawa, Y. Latitudinal Difference in the Species Richness of Photosymbiotic Ascidians Along the East Coast of Taiwan. Zool. Stud. 2020, 59, 19. [Google Scholar]
- Kim, H.K.; Chan, B.K.K.; Lee, S.-K.; Kim, W. Biogeography of intertidal and subtidal native and invasive barnacles in Korea in relation to oceanographic current ecoregions and global climatic changes. J. Mar. Biol. Assoc. UK 2020, 100, 1079–1091. [Google Scholar] [CrossRef]
- Gaino, E.; Pronzato, R.; Corriero, G.; Buffa, P. Mortality of commercial sponges: Incidence in two mediterranean areas. Bolletino Zoöl. 1992, 59, 79–85. [Google Scholar] [CrossRef]
- Rizzello, R.; Corriero, G.; Scalera Liaci, L.; Pronzato, R. Estinzione e ricolonizzazione di Spongia officinalis nello Stagnone di Marsala. Biol. Mar. Medit. 1997, 4, 443–444. [Google Scholar]
- Cerrano, C.; Bavestrello, G.; Bianchi, C.N.; Cattaneo-Vietti, R.; Bava, S.; Morganti, C.; Morri, C.; Picco, P.; Sara, G.; Schiaparelli, S.; et al. A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (Northwest- ern Mediterranean), summer 1999. Ecol. Lett. 2000, 3, 284–293. [Google Scholar] [CrossRef]
- Zocchi, E.; Basile, G.; Cerrano, C.; Bavestrello, G.; Giovine, M.; Bruzzone, S.; Guida, L.; Carpaneto, A.; Magrassi, R.; Usai, C. ABA- and cADPR-mediated effects on respiration and filtration downstream of the temperature-signaling cascade in sponges. J. Cell. Sci. 2003, 116, 629–636. [Google Scholar] [CrossRef] [Green Version]
- Pronzato, R.; Manconi, R. Mediterranean commercial sponges: Over 5000 years of natural history and cultural heritage. Mar. Ecol. 2008, 29, 146–166. [Google Scholar] [CrossRef]
- Bertolino, M.; Betti, F.; Bo, M.; Cattaneo-Vietti, R.; Pansini, M.; Romero, J.; Bavestrello, G. Changes and stability of a Mediterranean hard bottom benthic community over 25 years. J. Mar. Biol. Assoc. UK 2016, 96, 341–350. [Google Scholar] [CrossRef]
- Stabili, L.; Cardone, F.; Alifano, P.; Tredici, S.M.; Piraino, S.; Corriero, G.; Gaino, E. Epidemic mortality of the sponge Ircinia variabilis (Schmidth, 1862) associated to proliferation of a Vibrio bacterium. Microb. Ecol. 2012, 64, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.S. Sponge disease: A global threat? Environ. Microbiology 2007, 9, 1363–1375. [Google Scholar]
- Strusi, A.; Pastore, M. Osservazioni idrografiche nel Mar Grande e nel Mar Piccolo di Taranto. Campagna 1970–71. Oebalia 1975, 1, 1–64. [Google Scholar]
- Caroppo, C.; Cardellicchio, N.; Cavallo, R.A. Ciclo annuale del fitoplancton nei mari di Taranto: Influenza della qualità delle acque. Biol. Mar. Medit. 1994, 1, 201–206. [Google Scholar]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercurio, M.; Pierri, C.; Cardone, F.; Corriero, G. Temporal and Spatial Variations of Geodia cydonium (Jameson) (Porifera, Demospongiae) in the Mediterranean Confined Environments. Diversity 2021, 13, 615. https://doi.org/10.3390/d13120615
Mercurio M, Pierri C, Cardone F, Corriero G. Temporal and Spatial Variations of Geodia cydonium (Jameson) (Porifera, Demospongiae) in the Mediterranean Confined Environments. Diversity. 2021; 13(12):615. https://doi.org/10.3390/d13120615
Chicago/Turabian StyleMercurio, Maria, Cataldo Pierri, Frine Cardone, and Giuseppe Corriero. 2021. "Temporal and Spatial Variations of Geodia cydonium (Jameson) (Porifera, Demospongiae) in the Mediterranean Confined Environments" Diversity 13, no. 12: 615. https://doi.org/10.3390/d13120615
APA StyleMercurio, M., Pierri, C., Cardone, F., & Corriero, G. (2021). Temporal and Spatial Variations of Geodia cydonium (Jameson) (Porifera, Demospongiae) in the Mediterranean Confined Environments. Diversity, 13(12), 615. https://doi.org/10.3390/d13120615