Historical Zooplankton Composition Indicates Eutrophication Stages in a Neotropical Aquatic System: The Case of Lake Amatitlán, Central America
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling Methods
2.1.1. Species Richness
2.1.2. Species Abundance and Abiotic Variables
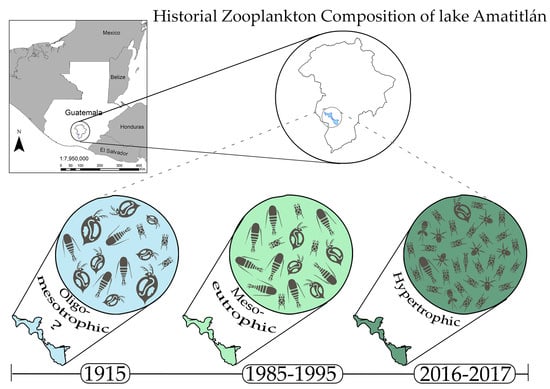
2.2. Historical and Actual Records of Zooplankton and Environmental Parameters Analysis
3. Results
3.1. Species Richness
3.2. Species Abundance
3.3. Environmental Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- José de Paggi, S.B.; Wallace, R.; Fontaneto, D.; Marinone, M.C. Phylum Rotifera. In Thorp and Covich’s Freshwater Invertebrates; Damborenea, C., Rogers, C.D., James, T., Eds.; Elsevier Science Publishing Co Inc.: San Diego, CA, USA, 2020; pp. 145–200. [Google Scholar]
- Cervantes-Martínez, A.; Gutiérrez-Aguirre, M.A. Physicochemistry and Zooplankton of Two Karstic Sinkholes in the Yucatan Peninsula, Mexico. J. Limnol. 2015, 74, 382–393. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Morales, E.; Reid, J.W.; Ilige, T.; Fiers, F. Catálogo de Los Copépodos (Crustacea) Continentales de La Península de Yucatán, México; Chetumal, Quintana Roo, México; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Ciudad de México, Mexico, 1996; 296p. [Google Scholar]
- Gómez-Márquez, J.L.; Peña-Mendoza, B.; Guzmán-Santiago, J.L.; Gallardo-Pineda, V. Composición, Abundancia Del Zooplancton y Calidad de Agua En Un Microreservorio En El Estado de Morelos. Hidrobiologica 2013, 23, 227–240. [Google Scholar]
- Gama Flores, J.L.; Sarma, S.S.S.; López Rocha, A.N.; Nandini, S. Effects of Cladoceran-Conditioned Medium on the Demography of Brachionid Rotifers (Rotifera: Brachionidae). Hydrobiologia 2018, 844, 21–30. [Google Scholar] [CrossRef]
- Uye, S.I. Replacement of Large Copepods by Small Ones with Eutrophication of Embayments: Cause and Consequence. Hydrobiologia 1994, 292–293, 513–519. [Google Scholar] [CrossRef]
- Basterrechea-Díaz, M. El Lago de Amatitlán: Década de Estudios Limnológicos 1985–1995; Academia de Ciencias Médicas, Físicas y Naturales de Guatemala: Guatemala City, Guatemala, 1997; 45p. [Google Scholar]
- Sigui, N. ¿Por Qué Continúa La Contaminación de Aguas En Guatemala? Cienc. Tecnol. Salud 2016, 3, 167–176. [Google Scholar] [CrossRef]
- Rodas-Pernillo, E.; Vasquez-Moscoso, C.A.; García, O.F. Dinámica Del Consumo y Aporte de Nutrientes de Fitoplancton, Dominado Por Microcystis sp. (Cyanophyceae) Del Lago de Amatitlán. Cienc. Tecnol. Salud 2020, 7, 2409–3459. [Google Scholar]
- Richardson-Varas, R.; Muñoz-Luza, M.; Landeros-Cáceres, F.; Contreras-Celis, V.; Carranza-González, J.; Nuñez, M.; Hernández, E.; Alfonso-Álvarez, R.; Ramos, E.; Mazariegos, J.; et al. Índice de Fragilidad Ambiental En Las Cuencas Hidro-Geomorfológicas Del Lago Peñuelas, Chile y Del Lago Amatitlán, Guatemala. Rev. Geográfica 2015, 156, 98–109. [Google Scholar]
- Brandorff, G.O. Distribution of Some Calanoida (Crustacea: Copepoda) from the Yucatán Peninsula, Belize and Guatemala. Rev. Biol. Trop. 2012, 60, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Martínez, A.; Elías-Gutiérrez, M.; Suárez-Morales, E. Limnological and Morphometrical Data of Eight Karstic Systems “cenotes” of the Yucatan Peninsula, Mexico, during the Dry Season (February–May, 2001). Hydrobiologia 2002, 482, 167–177. [Google Scholar] [CrossRef]
- Schmitter-Soto, J.J.; Comín, F.A.; Escobar-Briones, E.; Herrera-Silveira, J.; Alcocer, J.; Suárez-Morales, E.; Elías-Gutiérrez, M.; Díaz-Arce, V.; Marín, L.E.; Steinich, B. Hydrogeochemical and Biological Characteristics of Cenotes in the Yucatan Peninsula (SE Mexico). Hydrobiologia 2002, 467, 215–228. [Google Scholar] [CrossRef]
- Jiménez, S.; Juárez, J.; Trujillo, L.; Dubón, S.; Valenzuela, O.; Castro, A.M. Calidad de Agua de La Cuenca y Lago de Amatitlán; División de Control, Calidad Ambiental y Manejo de Lagos: Guatemala City, Guatemala, 2015; 32p. [Google Scholar]
- Elías-Gutiérrez, M.; Kotov, A.A.; Garfias-Espejo, T. Cladocera (Crustacea: Ctenopoda, Anomopoda) from Southern Mexico, Belize and Northern Guatemala, with Some Biogeographical Notes. Zootaxa 2006, 1119, 1–27. [Google Scholar] [CrossRef]
- García-Morales, A.E.; Elías-Gutiérrez, M. The Rotifer Fauna of Guatemala and Belize: Survey and Biogeographical Affinities. Rev. Biol. Trop. 2007, 55, 569–584. [Google Scholar]
- Pérez, L.; Bugja, R.; Lorenschat, J.; Brenner, M.; Curtis, J.; Hoelzmann, P.; Islebe, G.; Scharf, B.; Schwalb, A. Aquatic Ecosystems of the Yucatán Peninsula (Mexico), Belize, and Guatemala. Hydrobiologia 2011, 661, 407–433. [Google Scholar] [CrossRef]
- Cervantes-Martínez, A.; Elías-Gutiérrez, M.; Gutiérrez-Aguirre, M.A.; Kotov, A.A. Ecological Remarks on Mastigodiaptomus Nesus Bowman, 1986 (Copepoda: Calanoida) in a Mexican Karstic Sinkhole. Hydrobiologia 2005, 542, 95–102. [Google Scholar] [CrossRef]
- Ramírez García, P.; Nandini, S.; Sarma, S.S.S.; Robles Valderrama, E.; Cuesta, I.; Hurtado, M.D. Seasonal Varations of Zooplankton Abundance in the Freshwater Reservoir Valle de Bravo (Mexico). Hydrobiologia 2002, 467, 99–108. [Google Scholar] [CrossRef]
- Koste, W. Rotatoria: Die Rädertiere Mitteleuropas: Ein Bestimmunswerk Bengründet von Max Voigt Überordnung Monogononta; Gebrüder Borntraeger: Berlin, Germany; Stuttgart, Germany, 1978; 234p. [Google Scholar]
- Fontaneto, D.; De Smet, W.H. Rotifera. In Handbook of Zoology Gastrotricha, Cycloneuralia and Gnathifera; Rhaera, A.S., Ed.; De Gruyter-GmbH: Berlin, Germany, 2015; Volume 3, pp. 196–217. [Google Scholar]
- Elías-Gutiérrez, M.; Suárez-Morales, E.; Gutiérrez-Aguirre, M.A.; Silva-Briano, M.; Granados-Ramírez, J.G.; Garfias-Espejo, T. Cladocera y Copepoda de Las Aguas Continentales de México. Guía Ilustrada; Universidad Autónoma de México: Ciudad de México, Mexico, 2008; 322p. [Google Scholar]
- Suarez-Morales, E.; Gutiérrez-Aguirre, M.A.; Gómez, S.; Perbiche-Neves, G.; Previattelli, D.; Dos Santos-Silva, N.; da Rocha, C.E.F.; Mercado-Salas, N.F.; Manriquez, T.M.; Cruz-Quintana, Y.; et al. Class Copepoda. In Thorp and Covich’s Freshwater Invertebrates: Volume 5: Keys to Neotropical and Antarctic Fauna; Damborenea, C., Rogers, C.D., James, T., Eds.; Elsevier Science Publishing Co Inc.: San Diego, CA, USA, 2020; pp. 663–796. [Google Scholar]
- Juday, C. Limnological Studies on Some Lakes in Central America. Wisconsin Acad. Sci. Arts Lett. 1915, 18, 214–250. [Google Scholar]
- Wilson, M.S. New Species and Distribution Records of Diaptomid Copepods from the Marsh Collection in the United States National Museum. J. Washingt. Acad. Sci. 1941, 31, 509–515. [Google Scholar]
- Brezonik, P.L.; Fox, J.L. The Limnology of Selected Guatemalan Lakes. Hydrobiologia 1974, 45, 467–487. [Google Scholar] [CrossRef]
- Ellenberg, R.L. Limnology of Lake Amatitlán in Guatemala and Its Eutrophication Process. Ph.D. Thesis, Technical University of Berlin, Berlin, Germany, 2014; 128p. [Google Scholar]
- Frutos, S.M.; Poi, A.S.G.; Neiff, J.J. Zooplankton Abundance and Species Diversity in Two Lakes with Different Trophic States (Corrientes, Argentina) Abundância e Diversidade Específica Do Zooplâncton Em Dois Lagos. Acta Limnol. Bras. 2009, 21, 367–375. [Google Scholar]
- Moreno-Gutiérrez, R.M.; Sarma, S.S.S.; Sobrino-Figueroa, A.S.; Nandini, S. Population Growth Potential of Rotifers from a High Altitude Eutrophic Waterbody, Madín Reseivoir (State of Mexico, Mexico): The Importance of Seasonal Sampling. J. Limnol. 2020, 77, 441–451. [Google Scholar] [CrossRef]
- Obertegger, U.; Smith, H.A.; Flaim, G.; Wallace, R.L. Using the Guild Ratio to Characterize Pelagic Rotifer Communities. Hydrobiologia 2011, 662, 157–162. [Google Scholar] [CrossRef]
- Reid, J.W. Arctodiaptomusdorsalis (Marsh): A Case History of Copepod Dispersal. Banisteria 2007, 1860, 3–18. [Google Scholar]
- Papa, R.D.S.; Li, H.; Tordesillas, D.T.; Han, B.; Dumont, H.J. Massive Invasion of Arctodiaptomus dorsalis (Copepoda, Calanoida, Diaptomidae) in Philippine Lakes: A Threat to Asian Zooplankton Biodiversity? Biol. Invasions 2012, 14, 2471–2478. [Google Scholar] [CrossRef]
- Metillo, E.B.; Masorong, A.M.; Macabangkit, S.A.N.; Licayan, J.R.U.; Tordesillas, D.T.; Papa, R.D.S. First Record of the Invasive Arctodiaptomus dorsalis (Marsh, 1907) (Copepoda: Calanoida: Diaptomidae) in Lake Lanao (Mindanao Is., Philippines). Acta Manila. Ser. A 2014, 62, 19–23. [Google Scholar]
- Perbiche-Neves, G.; Previattelli, D.; Pie, M.R.; Duran, A.; Suárez-Morales, E.; Boxshall, G.A.; Nogueira, M.G.; da Rocha, C.E.F. Historical Biogeography of the Neotropical Diaptomidae (Crustacea: Copepoda). Front. Zool. 2014, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Elías-Gutiérrez, M.; Jerónimo, F.M.; Ivanova, N.V.; Valdez-Moreno, M.; Hebert, P.D.N. DNA Barcodes for Cladocera and Copepoda from Mexico and Guatemala, Highlights and New Discoveries. Zootaxa 2008, 1839, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Aguirre, M.A.; Cervantes-Martínez, A.; Elías-Gutiérrez, M.; Lugo-Vázquez, A. Remarks on Mastigodiaptomus (Calanoida: Diaptomidae) from Mexico Using Integrative Taxonomy, with a Key of Identification and Three New Species. PeerJ 2020, 8, e8416. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Aguirre, M.A.; Suárez-Morales, E. The Eurasian Thermocyclops crassus (Fischer, 1853) (Copepoda, Cyclopoida) Found in Southeastern Mexico. Crustaceana 2000, 73, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Aguirre, M.A.; Reid, J.W.; Suárez-Morales, E. An Afro-Asian Species of Mesocyclops (Copepoda: Cyclopoida) in Central America and Mexico. J. Crustacean Biol. 2003, 23, 352–363. [Google Scholar] [CrossRef] [Green Version]
- Collado, C.; Defaye, D.; Dussart, B.H.; Fernando, C.H. The Freshwater Copepoda (Crustacea) of Costa Rica with Notes on Some Species. Hydrobiologia 1984, 119, 89–99. [Google Scholar] [CrossRef]
- Gutiérrez-Aguirre, M.A.; Suárez-Morales, E.; Cervantes-Martínez, A.; Elías-Gutiérrez, M.; Previattelli, D. The Neotropical Species of Mesocyclops (Copepoda, Cyclopoida): An Upgraded Identification Key and Comments on Selected Taxa. J. Nat. Hist. 2006, 40, 549–570. [Google Scholar] [CrossRef]
- Suárez-Morales, E.; Gutiérrez-Aguirre, M.A.; Elías-Gutiérrez, M. Observations on the Structure of Mandibular Gnathobase in Some American Mesocyclops (Copepoda: Cyclopidae). Proc. Biol. Soc. Wash. 2003, 116, 742–753. [Google Scholar]
- Cervantes-Martínez, A.; Gutiérrez-Aguirre, M.A.; Delgado-Blas, V.H.; Ruíz-Ramírez, J.-D. Especies de Zooplancton Dulceacuícola de Cozumel; Quintana Roo, Universidad de Quintana Roo (UQROO): Cozumel, Mexico; Chetumal, Mexico, 2018; 86p. [Google Scholar]
- Connolly, J.K.; Watkins, J.M.; Hinchey, E.K.; Rudstam, L.G.; Reid, J.W. New Cyclopoid Copepod (Thermocyclops crassus) Reported in the Laurentian Great Lakes. J. Great Lakes Res. 2017, 43, 198–203. [Google Scholar] [CrossRef]
- Verbitsky, V.B.; Lazareva, V.I.; Medyantseva, E.N.; Malysheva, O.A.; Zhdanova, S.M.; Verbitskaya, T.I.; Grishanin, A.K. The Preferred and Avoidance Temperatures of Thermocyclops crassus (Fischer, 1853) and Their Relation to the Temperature of Optimal, Pessimal and Normal Performance of the Species. J. Therm. Biol. 2018, 78, 106–113. [Google Scholar] [CrossRef] [PubMed]

| Species | Current Data | Historical Data | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Phylum: Rotifera Monogononta: Ploimida | ||||||||
| Family: Epiphanidae Harring, 1913 | ||||||||
| Epiphanesmacroura Barrois & Daday, 1894 * | x | x | - | - | - | - | - | - |
| Family: Brachionidae Ehrenberg, 1838 | - | - | - | - | - | - | - | - |
| Anuraeopsisfissa (Gosse, 1851) * | x | - | - | - | - | - | - | - |
| Brachionusangularis (Gosse, 1851) * | x | x | x | x | - | - | - | - |
| B. calyciflorus Pallas, 1766 * | x | x | x | x | - | - | - | - |
| B. plicatilis Müeller, 1786 * | x | - | - | x | - | - | - | - |
| B. havanaensis Rousselet, 1911 * | x | x | x | x | - | - | - | - |
| Keratella sp. | - | - | - | - | - | x | - | - |
| K. americana Carlin, 1943 * | x | x | x | x | - | - | - | - |
| K. cochleraris (Gosse, 1851) | - | - | - | - | - | - | - | x |
| Family: Trichocercidae Harring, 1913 | ||||||||
| Trichocerca cf. longiseta (Schrank, 1802) * | - | x | x | x | - | - | - | - |
| T. pusilla (Lauterborn, 1898) * | - | x | x | x | - | - | - | - |
| Family: Asplanchnidae Eckstein, 1883 | ||||||||
| Asplanchnasieboldi (Leydig, 1854) * | x | x | x | - | - | - | - | - |
| Flosculariaceae: Family: Trochosphaeridae Harring, 1913 | ||||||||
| Filinialongiseta (Ehrenberg, 1834) | x | x | x | x | - | - | - | x |
| F. terminalis (Plate, 1886) * | x | x | x | - | - | - | - | - |
| Subclass: Bdelloidea * | x | - | - | - | - | - | - | - |
| Superclass: Crustacea Brachiopoda: Cladocera: Anomopoda | ||||||||
| Family: Daphniidae Straus, 1820 | ||||||||
| Daphnia sp. | - | - | - | - | - | x | - | - |
| D. hyalina Leydig, 1860 | - | - | - | - | - | - | - | x |
| Ceriodaphnia sp. | x | - | - | - | - | x | - | x |
| C. lacustris Birge, 1893 | - | - | - | - | - | - | - | x |
| C. pulchella Sars, 1862 | - | - | - | - | - | - | - | x |
| Family: Bosminidae Sars, 1865 | ||||||||
| Bosmina sp. | - | - | - | - | - | x | - | - |
| Bosminalongirostris O. F. Müeller, 1776 | - | - | - | - | - | - | - | x |
| Family: Chydoridae Stebbing, 1902 | ||||||||
| Chydorussphaericus (O.F. Müeller, 1785) | - | - | - | - | - | - | - | x |
| Copepoda: Calanoida Family: Diaptomidae G.O. Sars, 1932 | ||||||||
| Subfamily: Diaptominae Kiefer, 1932 | ||||||||
| Arctodiaptomusdorsalis (Marsh, 1907) | x | - | - | - | x | - | - | - |
| Mastigodiaptomusalbuquerquensis (Herrick, 1895) | - | - | - | - | - | - | - | x |
| M. amatitlanensis (Wilson, 1941) | - | - | - | - | - | - | x | - |
| Copepoda: Cyclopoida Family: Cyclopidae Kiefer, 1927 | ||||||||
| Subfamily: Cyclopinae Kiefer, 1927 | ||||||||
| Thermocyclopscrassus (Fischer, 1853) * | - | x | - | - | - | - | - | - |
| Mesocyclopsthermocyclopoides Harada, 1931 * | x | - | - | - | - | - | - | - |
| Subfamily: Eucyclopinae Kiefer, 1927 | ||||||||
| Eucyclopsserrulatus (Fischer, 1851) | - | - | - | - | - | - | - | x |
| Nauplii | x | x | x | x | - | x | - | x |
| Juvenile Cyclopoid | x | x | x | x | - | - | - | - |
| Juvenile Calanoid | x | x | x | x | - | - | - | - |
| Species | Abundance (ind L−1) | |||
|---|---|---|---|---|
| EC | OC | BPO | MICH | |
| Brachionusangularis | 0.00 | 0.00 | 71.56 | 1.87 |
| Brachionuscalyciflorus | 0.70 | 0.70 | 85.56 | 4.67 |
| Brachionusplicatilis | 0.00 | 0.47 | 0.00 | 0.00 |
| Trichocerca cf. longiseta | 0.00 | 0.00 | 14.00 | 12.60 |
| Trichocercapusilla | 0.00 | 8.40 | 13.22 | 11.20 |
| Asplanchnasieboldi | 1.40 | 1.17 | 2.33 | 0.93 |
| Filinialongiseta | 0.00 | 1.40 | 28.00 | 13.07 |
| Filiniaterminalis | 8.17 | 49.23 | 35.00 | 60.20 |
| Brachionushavanaensis | 9.33 | 153.53 | 108.89 | 165.20 |
| Keratellaamericana | 121.80 | 432.60 | 265.22 | 408.33 |
| Nauplii | 3.50 | 2.57 | 3.11 | 1.40 |
| Juvenile Cyclopoid | 5.83 | 1.63 | 1.56 | 0.93 |
| Juvenile Calanoid | 2.80 | 0.47 | 3.89 | 0.47 |
| M. thermocyclopoides | 0.23 | 0.00 | 0.00 | 0.00 |
| Environmental Variables | 1910 [24] | 1969 [26] | 1985–1995 [7] | 2008–2013 [27] | 2016 *** | 2017 *** |
|---|---|---|---|---|---|---|
| pH | ND | 7.70 | 7.75 | 8.69 | 8.26 | 8.33 |
| Water temperature (°C) | 19.86 | ND | 22.75 | 25.00 | 24.46 | 24.23 |
| Conductivity (µS cm−1) | ND | 830 | 802 | 682 | 655.95 | 678.23 |
| TDS (mg L−1) | ND | ND | 610 | ND | 339.99 | 341.43 |
| Dissolved oxygen O2 (mg L−1) | 4.74 * | 8.40 ** | 4.20 | 8.90 | 4.76 | 4.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaime, S.; Cervantes-Martínez, A.; Gutiérrez-Aguirre, M.A.; Suárez-Morales, E.; Juárez-Pernillo, J.R.; Reyes-Solares, E.M.; Delgado-Blas, V.H. Historical Zooplankton Composition Indicates Eutrophication Stages in a Neotropical Aquatic System: The Case of Lake Amatitlán, Central America. Diversity 2021, 13, 432. https://doi.org/10.3390/d13090432
Jaime S, Cervantes-Martínez A, Gutiérrez-Aguirre MA, Suárez-Morales E, Juárez-Pernillo JR, Reyes-Solares EM, Delgado-Blas VH. Historical Zooplankton Composition Indicates Eutrophication Stages in a Neotropical Aquatic System: The Case of Lake Amatitlán, Central America. Diversity. 2021; 13(9):432. https://doi.org/10.3390/d13090432
Chicago/Turabian StyleJaime, Sarahi, Adrián Cervantes-Martínez, Martha A. Gutiérrez-Aguirre, Eduardo Suárez-Morales, Julio R. Juárez-Pernillo, Elena M. Reyes-Solares, and Victor H. Delgado-Blas. 2021. "Historical Zooplankton Composition Indicates Eutrophication Stages in a Neotropical Aquatic System: The Case of Lake Amatitlán, Central America" Diversity 13, no. 9: 432. https://doi.org/10.3390/d13090432
APA StyleJaime, S., Cervantes-Martínez, A., Gutiérrez-Aguirre, M. A., Suárez-Morales, E., Juárez-Pernillo, J. R., Reyes-Solares, E. M., & Delgado-Blas, V. H. (2021). Historical Zooplankton Composition Indicates Eutrophication Stages in a Neotropical Aquatic System: The Case of Lake Amatitlán, Central America. Diversity, 13(9), 432. https://doi.org/10.3390/d13090432