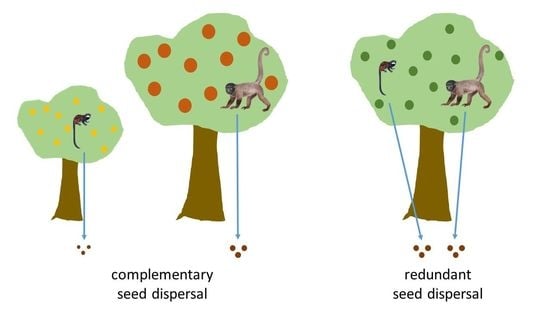
Small but Nice–Seed Dispersal by Tamarins Compared to Large Neotropical Primates
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Diversity of Consumed and Dispersed Fruit Species
3.2. Consistency of Seed Dispersal
3.3. Number of Seeds and Seed Species Dispersed per Defecation
3.4. Seed Size
3.5. Germination Success and Time
3.6. Seed Rain
3.7. Seed Dispersal Distances
3.8. Seed Dispersal Sites
3.9. Directed Dispersal
3.10. Dispersal into Secondary Forest
3.11. Effects on Plant Population Genetics
4. Concluding Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cousens, R.; Dytham, C.; Law, R. Dispersal in Plants. A Population Perspective; Oxford University Press: Oxford, UK, 2008; p. x+221. [Google Scholar]
- Degen, B.; Caron, H.; Bandou, E.; Maggia, L.; Chevallier, M.H.; Leveau, A.; Kremer, A. Fine-scale spatial genetic structure of eight tropical tree species as analysed by RAPDs. Heredity 2001, 87, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Terborgh, J.; Pitman, N.; Silman, M.; Schichter, H.; Núñez, P. Maintenance of tree diversity in tropical forests. In Seed Dispersal and Frugivory: Ecology, Evolution and Conservation; Levey, D.J., Silva, W.R., Galetti, M., Eds.; CABI: Wallingford, UK, 2002; pp. 1–17. [Google Scholar]
- van der Pijl, L. Principles of Dispersal in Higher Plants; Springer: Berlin/Heidelberg, Germany, 1982; p. x+215. [Google Scholar]
- Fleming, T.H.; Kresstt, W.J. The Ornaments of Life. Coevolution and Conservation in the Tropics; University of Chicago Press: Chicago, IL, USA, 2013; p. xii+588. [Google Scholar]
- Gentry, A.H. Patterns of Neotropical plant species diversity. Evol. Biol. 1982, 15, 1–84. [Google Scholar]
- Correa, D.F.; Stevenson, P.R.; Umaña, M.N.; de Souza Coelho, L.; Filho, D.D.A.L.; Salomão, R.P.; Amaral, I.L.D.; Wittmann, F.; Matos, F.D.D.A.; Castilho, C.V.; et al. Geographic patterns of tree dispersal modes in Amazonia and their ecological correlates. Global Ecol. Biogeogr. 2022. [Google Scholar] [CrossRef]
- Richard, A.F. Primates in Nature; W.H.Freeman and Company: New York, NY, USA, 1985; p. xii+558. [Google Scholar]
- Schupp, E.W. Quantity, quality, and the effectiveness of seed dispersal by animals. In Frugivory and Seed Dispersal: Ecological and Evolutionary Aspects; Fleming, T.H., Estrada, A., Eds.; Kluwer Academic Press: Dordrecht, Netherlands, 1993; pp. 15–29. [Google Scholar]
- Schupp, E.W.; Jordano, P.; Gómez, J.M. Seed dispersal effectiveness revisited: A conceptual review. New Phytol. 2010, 188, 333–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuzessy, L.; Sobral, G.; Culot, L. Linking howler monkey ranging and defecation patterns to primary and secondary seed dispersal. Am. J. Primatol. 2022, 84, e23354. [Google Scholar] [CrossRef]
- Rosenberger, A.L.; Strier, K.B. Adaptive radiation of the ateline primates. J. Hum. Evol. 1989, 18, 717–750. [Google Scholar] [CrossRef]
- Smith, R.F.; Jungers, W.L. Body mass in comparative primatology. J. Hum. Evol. 1997, 32, 523–559. [Google Scholar] [CrossRef]
- Rylands, A.B.; Heymann, E.W.; Alfaro, J.L.; Buckner, J.C.; Roos, C.; Matauschek, C.; Boubli, J.P.; Sampaio, R.; Mittermeier, R.A. Taxonomic review of the New World tamarins (Primates: Callitrichidae). Zool. J. Linn. Soc. 2016, 177, 1003–1028. [Google Scholar] [CrossRef]
- Di Fiore, A.; Link, A.; Dew, J.L. Diets of wild spider monkeys. In Spider Monkeys. Behavior, Ecology and Evolution of the Genus Ateles; Campbell, C.J., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 81–137. [Google Scholar]
- Dew, J.L. Foraging, food choice, and food processing by sympatric ripe-fruit specialists: Lagothrix lagotricha poeppigii and Ateles belzebuth belzebuth. Int. J. Primatol. 2005, 26, 1053–1075. [Google Scholar] [CrossRef]
- Peres, C.A. Diet and feeding ecology of saddle-back (Saguinus fuscicollis) and moustached (S. mystax) tamarins in Amazonian terra firme forest. J. Zool. Lond. 1993, 230, 567–592. [Google Scholar]
- Porter, L.M. Dietary differences among sympatric Callitrichinae in northern Bolivia: Callimico goeldii, Saguinus fuscicollis and S. labiatus. Int. J. Primatol 2001, 22, 961–992. [Google Scholar] [CrossRef]
- Culot, L. Primary Seed Dispersal by Two Sympatric Species of Tamarins, Saguinus Fuscicollis and Saguinus Mystax, and Post-Dispersal Seed Fate. Ph. D. Thesis, Université de Liège, Liège, Belgium, 2009. [Google Scholar]
- Knogge, C. Tier-Pflanze-Interaktionen im Amazonas-Regenwald: Samenausbreitung durch Die Sympatrischen Tamarinarten Saguinus Mystax und Saguinus Fuscicollis (Callitrichinae, Primates); Schüling Verlag: Münster, Germany, 1999; p. 151. [Google Scholar]
- Stevenson, P.R.; Castellanos, M.C.; Pizarro, J.C.; Garavito, M. Effects of seed dispersal by three ateline monkey species on seed germination at Tinigua National Park, Colombia. Int. J. Primatol. 2002, 23, 1187–1204. [Google Scholar] [CrossRef]
- van Roosmalen, M.G.M. Habitat preferences, diet, feeding strategy and social organization of the black spider monkey (Ateles paniscus paniscus Linnaeus 1758) in Surinam. Acta Amaz. 1985, 15, 1–238. [Google Scholar] [CrossRef] [Green Version]
- Defler, T.R.; Defler, S.B. Diet of a group of Lagothrix lagothricha lagothricha in southeastern Colombia. Int. J. Primatol. 1996, 17, 161–190. [Google Scholar] [CrossRef]
- Culot, L.; Muñoz Lazo, F.J.J.; Poncin, P.; Huynen, M.C.; Heymann, E.W. Seasonal variation in seed dispersal by tamarins alters seed rain in a secondary rainforest. Int. J. Primatol. 2010, 31, 553–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knogge, C.; Heymann, E.W. Seed dispersal by sympatric tamarins, Saguinus mystax and Saguinus fuscicollis: Diversity and characteristics of plant species. Folia Primatol. 2003, 74, 33–47. [Google Scholar] [CrossRef]
- Link, A.; Di Fiore, A. Seed dispersal by spider monkeys and its importance in the maintenance of neotropical rain-forest diversity. J. Trop. Ecol. 2006, 22, 235–246. [Google Scholar] [CrossRef]
- González, M.; Stevenson, P.R. Seed dispersal by woolly monkeys (Lagothrix lagothricha) at Caparú Biological Station (Colombia): Quantitative description and qualitative analysis. In The Woolly Monkey; Defler, T.R., Stevenson, P.R., Eds.; Springer: New York, NY, USA, 2014; pp. 147–165. [Google Scholar]
- Stevenson, P.R. Estimates of the number of seeds dispersed by a population of primates in a lowland forest in western Amazonia. In Seed Dispersal: Theory and Its application in a Changing World; Dennis, A.J., Schupp, E.W., Green, R.J., Westcott, D.A., Eds.; CABI International: Wallingford, UK, 2007; pp. 340–362. [Google Scholar]
- Dew, J.L. Spider monkeys as seed dispersers. In Spider Monkeys. Behavior, Ecology and Evolution of the Genus Ateles; Campbell, C.J., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 155–182. [Google Scholar]
- Palma, A.C.; Stevenson, P.R. Dispersión de semillas por monos araña en la Estación Biológica Cocha Cashu, Parque Nacional Manu, Perú. In Reporte Manu 2013; Groenendijk, J., Tovar, A., Eds.; San Diego Zoo Global Peru: Lima, Peru, 2013; pp. 178–195. [Google Scholar]
- de Luna, G.A.; García-Morera, Y.; Link, A. Behavior and ecology of the white-footed tamarin (Saguinus leucopus) in a fragmented landscape of Colombia: Small bodied primates and seed dispersal in Neotropical forests. Trop. Conserv. Sci. 2016, 9, 788–808. [Google Scholar] [CrossRef]
- Yumoto, T.; Kimura, K.; Nishimura, A. Estimation of the retention time and distances of seed dispersed by two monkey species, Alouatta seniculus and Lagothrix lagotricha, in a Colombian forest. Ecol. Res. 1999, 14, 179–191. [Google Scholar] [CrossRef]
- Stevenson, P.R. Pulp–seed attachment is a dominant variable explaining legitimate seed dispersal: A case study on woolly monkeys. Oecologia 2011, 166, 693–701. [Google Scholar] [CrossRef]
- Hershkovitz, P. Living New World monkeys (Platyrrhini); University of Chicago Press: Chicago, IL, USA, 1977; Volume 1, p. xiv+1117. [Google Scholar]
- Andresen, E. Seed dispersal by monkeys and the fate of dispersed seeds in a Peruvian rain forest. Biotropica 1999, 31, 145–158. [Google Scholar] [CrossRef]
- Stevenson, P.R.; Pineda, M.; Samper, T. Influence of seed size on dispersal patterns of woolly monkeys (Lagothrix lagothricha) at Tinigua Park, Colombia. Oikos 2005, 110, 435–440. [Google Scholar] [CrossRef]
- Ramírez, M.A.; Galvis, N.F.; Vargas, S.A.; Léon, J.J.; Cifuentes, E.F.; Stevenson, P.R. Seed dispersal by woolly monkeys in Cueva de los Guacharos National Park (Colombia): An Amazonian primate dispersing montane plants. In High Altitude Primates; Grow, N.B., Gursky-Doyen, S., Krzton, A., Eds.; Springer: New York, NY, USA, 2014; pp. 103–114. [Google Scholar]
- Garber, P.A.; Kitron, U. Seed swallowing in tamarins: Evidence of a curative function or enhanced foraging efficiency? Int. J. Primatol. 1997, 18, 523–538. [Google Scholar] [CrossRef]
- Oliveira, A.C.M.; Ferrari, S.F. Seed dispersal by black-handed tamarins, Saguinus midas niger (Callitrichinae, Primates): Implications for the regeneration of degraded forest habitats in eastern Amazonia. J. Trop. Ecol. 2000, 16, 709–716. [Google Scholar] [CrossRef]
- Fuzessy, L.F.; Cornelissen, T.G.; Janson, C.; Silveira, F.A.O. How do primates affect seed germination? A meta-analysis of gut passage effects on neotropical plants. Oikos 2016, 125, 1069–1080. [Google Scholar]
- Nunes, A. Um teste de germinaçao em sementes dispersas por macacos-Aranha em Maracá, Roraima; Brasil. Stud. Neotrop. Fauna Environ. 1995, 30, 31–36. [Google Scholar] [CrossRef]
- Stevenson, P.R. Seed dispersal by woolly monkeys (Lagothrix lagothricha) at Tinigua National Park, Colombia: Dispersal distance, germination rates, and dispersal quantity. Am. J.Primatol. 2000, 50, 275–289. [Google Scholar] [CrossRef]
- Knogge, C.; Herrera, E.R.T.; Heymann, E.W. Effect of passage through tamarin guts on the germination potential of dispersed seeds. Int. J. Primatol. 2003, 24, 1121–1128. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Wang, L.-X. Fruit consumption and seed dispersal of Ziziphus cinnamomum (Rhamnaceae) by two sympatric primates (Cebus apella and Ateles paniscus). Biotropica 1995, 27, 397–401. [Google Scholar] [CrossRef]
- Heymann, E.W.; Culot, L.; Knogge, C.; Piña, T.E.N.; Herrera, E.R.T.; Klapproth, M.; Zinner, D. Long-term consistency in spatial patterns of primate seed dispersal. Ecol. Evol. 2017, 7, 1435–1441. [Google Scholar] [CrossRef]
- Garber, P.A. The ecology of seed dispersal in two species of callitrichid primates (Saguinus mystax and Saguinus fuscicollis). Am. J.Primatol. 1986, 10, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Lazo, F.J.J.M.; Culot, L.; Huynen, M.C.; Heymann, E.W. Effect of resting patterns of tamarins (Saguinus fuscicollis and Saguinus mystax) on the spatial distribution of seeds and seedling recruitment. Int. J. Primatol. 2011, 32, 223–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldmann, M. Samenökologie von Parkia im Amazonas-Regenwald. Samenausbreitung durch Krallenaffen (Callitrichidae), Sekundäre Ausbreitung von Samen und Samenprädation. Diploma Thesis, Georg-August-Universität Göttingen, Göttingen, Germany, 2000. [Google Scholar]
- Karubian, J.; Ottewell, K.; Link, A.; Di Fiore, A. Genetic consequences of seed dispersal to sleeping trees by white-bellied spider monkeys. Acta Oecol. 2015, 68, 50–58. [Google Scholar] [CrossRef]
- Howe, H.F.; Smallwood, J. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Knogge, C.; Heymann, E.W.; Herrera, E.R.T. Seed dispersal of Asplundia peruviana (Cyclanthaceae) by the primate Saguinus fuscicollis. J. Trop. Ecol. 1998, 14, 99–102. [Google Scholar] [CrossRef]
- Garber, P.A. A comparative study of positional behavior in three species of tamarin monkeys. Primates 1991, 32, 219–230. [Google Scholar] [CrossRef]
- Nyakatura, J.A.; Heymann, E.W. Effects of support size and orientation on symmetric gaits in free-ranging tamarins of Amazonian Peru: Implications for the functional significance of primate gait sequence patterns. J. Hum. Evol. 2010, 58, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Defler, T.R. Locomotion and posture in Lagothrix lagotricha. Folia Primatol. 1999, 70, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Fleagle, J.G.; Mittermeier, R.A. Locomotor behavior, body size, and comparative ecology of seven Surinam monkeys. Am. J. Phys. Anthropol. 1980, 52, 301–314. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Fleagle, J.G. The locomotor and postural repertoires of Ateles geoffroyi and Colobus guereza, and a reevaluation of the locomotor category semibrachiation. Am. J. Phys. Anthropol. 1976, 45, 235–256. [Google Scholar] [CrossRef]
- Rylands, A.B. Habitat and the evolution of social and reproductive behavior in Callitrichidae. Am. J Primatol. 1996, 38, 5–18. [Google Scholar] [CrossRef]
- Stevenson, P.R.; Quiñones, M.J.; Ahumada, J.A. Ecological strategies of woolly monkeys (Lagothrix lagotricha) at Tinguana National Park, Colombia. Am. J Primatol. 1994, 32, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.B. Factors influencing spider monkey habitat use and ranging patterns. In Spider Monkeys: Behavior, Ecology and Evolution of the Genus Ateles; Campbell, C.J., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 138–154. [Google Scholar]
- Peres, C.A.; Dolman, P.M. Density compensation in neotropical primate communities: Evidence from 56 hunted and nonhunted Amazonian forests of varying productivity. Oecologia 2000, 122, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Heymann, E.W.; Culot, L.; Knogge, C.; Smith, A.C.; Herrera, E.R.T.; Müller, B.; Stojan-Dolar, M.; Ferrer, Y.L.; Kubisch, P.; Kupsch, D.; et al. Small Neotropical primates promote the natural regeneration of anthropogenically disturbed areas. Sci. Rep. 2019, 9, 10356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialozyt, R.; Luettmann, K.; Michalczyk, I.M.; Saboya, P.P.P.; Ziegenhagen, B.; Heymann, E.W. Primate seed dispersal leaves spatial genetic imprint throughout subsequent life stages of the Neotropical tree Parkia panurensis. Trees 2014, 28, 1569–1575. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, L.F.; Simonetti, J.A. Genetic structure of a mimosoid tree deprived of its seed disperser, the spider monkey. Conserv. Biol. 2000, 14, 1766–1775. [Google Scholar] [CrossRef]
- Culot, L.; Mann, C.; Lazo, F.J.J.M.; Huynen, M.C.; Heymann, E.W. Tamarins and dung beetles: An efficient diplochorous dispersal system in the Peruvian Amazonia. Biotropica 2011, 43, 84–92. [Google Scholar] [CrossRef]
- Santos-Heredia, C.; Andresen, E.; Zárate, D.A. Secondary seed dispersal by dung beetles in a Colombian rain forest: Effects of dung type and defecation pattern on seed fate. J. Trop. Ecol. 2010, 26, 355–364. [Google Scholar] [CrossRef]



| Primate Species | Number of Plant Species Exploited for Pulp | Number of Dispersed Plant Species | % Dispersed | Study Periods and Duration c | Source |
|---|---|---|---|---|---|
| Ateles belzebuth | 90 | 83 | 1992, duration not available | [21] | |
| Ateles belzebuth | 73 | 72 | 99 | Aug 1995–Jul 1996, 457.45 h | [16] |
| Ateles paniscus | 151 | 138 | 91 | Apr 1977–May 1978, 135 d, 865 h | [22] |
| Lagothrix l. lagothricha | 183 | 165 | 90 a | Jan 1985–Sep 1987, 2400 h | [23] |
| Lagothrix l. lugens | 90 | 84 | 1996–97, 720 h | [21] | |
| Lagothrix l. poeppigii | 104 | 88 | 95 | Aug 1995–Jul 1996, 429,45 h | [16] |
| Leontocebus nigrifrons, 1994–95 | 124 | 81 | 65 | Mar 1994–May 1995, 74 d | [20] |
| Leontocebus nigrifrons, 2005–08 | 251 | 154 | 61 | Sep 2005–Feb 2006, Jun–Nov 2006, March–Aug 2007, Dec 2007–May 2008, 2303 h | [24] |
| Saguinus mystax, 1994–95 | 130 | 67 | 52 | Mar 1994–May 1995, 67 d | [16] |
| Saguinus mystax, 2005–08 | 267 | 151 | 57 | Sep 2005–Feb 2006, Jun–Nov 2006, March–Aug 2007, Dec 2007–May 2008, 2303 h | [24] |
| L. nigrifrons + S. mystax combined, 1994–95 b | 155 | 88 | 57 | [16] | |
| L. nigrifrons + S. mystax combined, 2005–08 b | 307 | 166 | 54 | [24] |
| Primate Species | Parameter | Number of Seeds/ Defecation | Range | Sample Size (Number of Fecal Samples) | Source |
|---|---|---|---|---|---|
| Ateles belzebuth | median a mean a median b mean b | 7 39.1 5 7.8 | 0–402 | 738 | [26] |
| Ateles chamek | mean mean b | 249 22 | 89 | [30] | |
| Lagothrix l. lagothricha | Mean | 68 | 0–3905 | 1397 | [27] |
| Lagothrix l. lugens | Mean | 79 | 0–1409 | 1562 | [28] |
| Leontocebus nigrifrons | median | 1 | 1–4800+ | 1137 | Knogge & Heymann, unpubl. data |
| mean mean b | 2.2 1.7 | 1-4800+ | 1137 | [25] | |
| Saguinus mystax | median | 1 | 1–4800+ | 924 | Knogge & Heymann, unpubl. data |
| mean mean b | 2.2 1.5 | 1-4800+ | 924 | [25] | |
| Saguinus leucopus | 1–504 | 134 | [31] |
| Primate Species | Parameter | Number of Seed Species/ Defecation | Range | Sample Size (Number of Fecal Samples) | Source |
|---|---|---|---|---|---|
| Ateles belzebuth | mean ± SD a | 1.9 ± 1.3 | 0–7 | 738 | [26] |
| Ateles chamek | mean | 2.2 | 89 | [30] | |
| Lagothrix l. lagothricha | mean ± SD median b | 1.8 ± 1.0 2 | 0–5 | 176 | [32] |
| Lagothrix l. lagothricha | mean ± SD | 2.4 ± 1.3 | 0–9 | 1397 | [27] |
| Lagothrix l. lugens | mean | 2.5 | 0–9 | 1562 | [28] |
| Leontocebus nigrifrons | median | 1 | 1137 | Knogge & Heymann, unpubl. data | |
| mean | 1.2 | 1137 | [25] | ||
| Saguinus mystax | median | 1 | 924 | Knogge & Heymann, unpubl. data | |
| mean | 1.2 | 924 | [25] |
| Dimension | Parameter | Size [cm] | Range | Sample Size [Number of Species] | Source | |
|---|---|---|---|---|---|---|
| Ateles belzebuth | length | <0.1–3.9 | 133 | [26] | ||
| length | <0.1–3.2 | 71 | [21] | |||
| Ateles paniscus | length width | mean mean | 1.38 1.02 | 0.01–2.76 0.01–2.00 | 12 | [35] |
| Lagothrix l. lagothricha | length width | 0.8–2.13 0.62–1.48 | 11 | [32] | ||
| Lagothrix l. lagothricha | length | <0.1–4.00 | 80 | [21] | ||
| Lagothrix l. lagothricha | length width | median median | 1.10 | <0.1–3.30 <0.1–1.80 | n.a. n.a. | [36] |
| Lagothrix l. lugens | width | 0.07–2 | 28 | [37] | ||
| Leontocebus nigrifrons | lengthwidth | median median | 1.38 0.80 | 75 | Knogge & Heymann, unpubl. data | |
| length width | mean mean | 1.24 ± 0.56 0.75 ± 0.31 | 0.06–2.33 0.06–1.35 | 75 | [25] | |
| Saguinus mystax | length width | median median | 1.380.80 | 65 | Knogge & Heymann, unpubl. data | |
| length width | mean mean | 1.25 ± 0.59 0.74 ± 0.32 | 0.06–2.35 0.06–1.31 | 65 | [25] | |
| length width | mean mean | 1.19 ± 0.23 0.65 ± 0.10 | 0.73–1.95 0.45–0.97 | n.a. | [38] | |
| Saguinus geoffroyi | length width | mean mean | 1.12 ± 0.41 0.62 ± 0.20 | 0.26–2.3 0.24–1.2 | n.a. | [38] |
| Saguinus leucopus | length?b | mean | 1.1 | <0.1–2.6 | 44 | [31] |
| Saguinus niger | length width | 0.5–1.7 a 0.2–1.1 a | 6 | [39] |
| Primate Species | Measure | Total Number of Plant Species | Number of Species Enhanced a | Number of Species Reduced b | Number of Neutral Effects | Source |
|---|---|---|---|---|---|---|
| Ateles belzebuth | Success Time | 15 12 | 6 1 | 4 2 | 5 9 | [21] c |
| Success Time | 10 10 | 4 8 | 0 2 | 6 0 | [41] c | |
| Lagothrix l. lagothricha | Success Time | 16 9 | 8 1 | 1 3 | 7 5 | [21] c |
| Lagothrix l. lugens | Success | 21 | 8 | 1 | 12 | [42] d |
| Leontocebus nigrifrons | Success Time | 27 27 | 5 3 | 6 2 | 16 22 | [43] c,e |
| Saguinus mystax | Success Time | 24 24 | 3 7 | 3 0 | 18 17 | [43] c,e |
| Primate Species | Number of Seeds Dispersed /Year/ha a | Source |
|---|---|---|
| Ateles belzebuth | 13,500 | [26] |
| 2400 b | [29] | |
| Lagothrix l. lagothricha | 255,900 c 10,700 d | [27] |
| Lagothrix l. lugens | 91,250 e | [42] |
| 3,884,700 f | [28] | |
| Lagothrix l. poeppigii | 3200 b | [29] |
| Leontocebus nigrifrons | 21,900 | [20] |
| Saguinus mystax | 27,100 | [20] |
| L. nigrifrons + S. mystax combined g | 49,000 | [20] |
| Primate Species | Parameter | Dispersal Distance [m] | Maximal Dispersal Distance [m] | Sample Size (Number of Dispersal Events) | Source |
|---|---|---|---|---|---|
| Ateles belzebuth | mean ± SD median | 443 ± 334 318 | 1281 | 186 | [26] |
| Ateles paniscusa | mean ± SD | 252 ± 145 | 116 | [44] | |
| Lagothrix l. lagothricha | mean b | 377 | 1106 | 98 | [32] |
| Lagothrix l. lagothricha | mean | 577 | 1540 | 66 | [27] |
| Lagothrix l. lugens | mean ± SD | 354 ± 213 | 989 (1466 c) | 264 | [42] |
| Leontocebus nigrifrons, 1994–95 | median | 173 | 638 | 1059 | [45] |
| Leontocebus nigrifrons, 2005–08 | median | 183 | 593 | 349 | [45] |
| Saguinus mystax, 1994–95 | median | 152 | 709 | 817 | [45] |
| Saguinus mystax, 2005–08 | median | 160 | 585 | 301 | [45] |
| Leontocebus nigrifrons/ Saguinus mystax | 513 | 39 | [46] | ||
| Saguinus leucopus | mean ± SD | 206 ± 95 | 529 | 51 | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heymann, E.W.; Fuzessy, L.; Culot, L. Small but Nice–Seed Dispersal by Tamarins Compared to Large Neotropical Primates. Diversity 2022, 14, 1033. https://doi.org/10.3390/d14121033
Heymann EW, Fuzessy L, Culot L. Small but Nice–Seed Dispersal by Tamarins Compared to Large Neotropical Primates. Diversity. 2022; 14(12):1033. https://doi.org/10.3390/d14121033
Chicago/Turabian StyleHeymann, Eckhard W., Lisieux Fuzessy, and Laurence Culot. 2022. "Small but Nice–Seed Dispersal by Tamarins Compared to Large Neotropical Primates" Diversity 14, no. 12: 1033. https://doi.org/10.3390/d14121033
APA StyleHeymann, E. W., Fuzessy, L., & Culot, L. (2022). Small but Nice–Seed Dispersal by Tamarins Compared to Large Neotropical Primates. Diversity, 14(12), 1033. https://doi.org/10.3390/d14121033