Foraging Patterns of Non-Territorial Eastern Imperial Eagle (Aquila heliaca): A Case of Successful Adaptation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analyses
3. Results
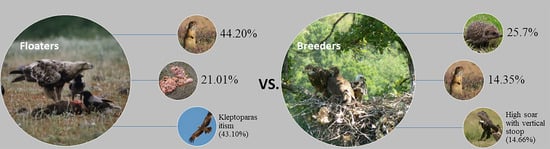
3.1. Diet Diversity and Comparison of Floaters vs. Breeders
| Prey | Observation (ind.) | Food Remains (ind.) | Total (ind.) | Total (%) |
|---|---|---|---|---|
| Spermophilus citellus | 50 | 11 | 61 | 44.20 |
| Rodentia (excl. Souslik) | 10 | 10 | 7.25 | |
| Columba livia f. domestica | 1 | 8 | 9 | 6.52 |
| Lepus europaeus | 5 | 5 | 3.62 | |
| Serpentes | 1 | 1 | 2 | 1.45 |
| Falco tinnunculus | 1 | 1 | 0.72 | |
| Carrion | 29 | 29 | 21.01 | |
| Unidentified | 14 | 14 | 10.14 | |
| Corvus frugilegus | 4 | 4 | 2.90 | |
| Erinaceus roumanicus | 1 | 1 | 0.72 | |
| Canis aureus | 2 | 2 | 1.45 | |
| Total | 118 | 20 | 138 | 100 |
3.2. Foraging Pattern of Non-Territorial EIEs
3.3. Foraging Mode and Success
3.4. Effect of Habitat Type, Prey Type, and Season
3.5. Effect of Eagle Age
4. Discussion
4.1. Dietary Diversification as a Case of Successful Adaptation
4.2. Factors Influencing Foraging Behavior and Success
4.3. Conservation Suggestion and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pyke, G.; Pulliam, H.R.; Charnov, E.L. Optimal Foraging: A Selective Review of Theory and Tests. Q. Rev. Biol. 1977, 52, 137–154. [Google Scholar] [CrossRef] [Green Version]
- Stephens, D.W.; Krebs, J.R. Foraging Theory; Princeton University Press: Princeton, NJ, USA, 1986. [Google Scholar]
- Stephens, D.W.; Brown, J.S.; Ydenberg, R.C. (Eds.) Foraging: Behavior and Ecology; University of Chicago Press: Chicago, IL, USA, 2008. [Google Scholar]
- Kramer, D.L. ‘Foraging Behavior’. In Evolutionary Ecology: Concepts and Case Studies; Fox, C.W., Roff, D.A., Fairbairn, D.J., Eds.; Oxford Academic: Chenango, NY, USA, 2001. [Google Scholar] [CrossRef]
- Sinervo, B. Optimal Foraging Theory: Constraints and Cognitive Processes. In Behavioral Ecology; Chapter 6; University of Santa Cruz: Santa Cruz, CA, USA, 1997; pp. 105–130. [Google Scholar]
- Naef-Daenzer, L.; Naef-Daenzer, B.; Nager, R.G. Prey selection and foraging performance of breeding Great Tits Parus major in relation to food availability. J. Avian Biol. 2000, 31, 206–214. [Google Scholar] [CrossRef]
- Moleón, M.; Sánchez-Zapata, J.A.; Real, J.; García-Charton, J.A.; Gil-Sánchez, J.M.; Palma, L.; Bautista, J.; Bayle, P. Large-scale spatio-temporal shifts in the diet of a predator mediated by an emerging infectious disease of its main prey. J. Biogeogr. 2009, 36, 1502–1515. [Google Scholar] [CrossRef]
- Gryz, J.; Krauze-Gryz, D. Food Niche Overlap of Avian Predators (Falconiformes, Strigiformes) in a Field and Forest Mosaic in Central Poland. Animals 2021, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Clouet, M.; Gerard, J.-F.; Goar, J.-L.; Goulard, M.; González, L.; Rebours, I.; Faure, C. Diet and Breeding Performance of the Golden Eagle Aquila Chrysaetos at the Eastern and Western Extremities of the Pyrenees: An Example of Intra-Population Variability. Ardeola 2017, 64, 347–361. [Google Scholar] [CrossRef]
- Demerdzhiev, D.; Boev, Z.; Dobrev, D.; Terziev, N.; Nedyalkov, N.; Stoychev, S.; Petrov, T. Diet of Eastern Imperial Eagle (Aquila heliaca) in Bulgaria: Composition, distribution and variation. Biodivers. Data J. 2022, 10, e77746. [Google Scholar] [CrossRef]
- Horváth, M.; Szitta, T.; Firmánszky, G.; Solti, B.; Kovács, A.; Moskát, C. Spatial variation in prey composition and its possible effect on reproductive success in an expanding Eastern Imperial Eagle (Aquila heliaca) population. Acta Zool. Acad. Sci. Hung. 2010, 56, 187–200. [Google Scholar]
- Katzner, T.; Bragin, E.; Knick, S.; Smith, A. Relationship between demographics and diet specificity of Eastern Imperial Eagle Aquila heliaca in Kazakhstan. IBIS 2005, 147, 576–586. [Google Scholar] [CrossRef]
- Lourenço, R.; Delgado, M.D.M.; Campioni, L.; Korpimäki, E.; Penteriani, V. Evaluating the influence of diet-related variables on breeding performance and home range behaviour of a top predator. Popul. Ecol. 2015, 57, 625–636. [Google Scholar] [CrossRef] [Green Version]
- Murgatroyd, M.; Avery, G.; Underhill, L.; Amar, A. Data from: Adaptability of a specialist predator: The effects of land use on diet diversification and breeding performance of Verreaux’s eagles. J. Avian Biol. 2016, 47, 001–012. [Google Scholar] [CrossRef]
- Caro, J.; Ontiveros, D.; Pleguezuelos, J.M. The feeding ecology of Bonelli’s eagle (Aquila fasciata) floaters in southern Spain: Implications for conservation. Eur. J. Wildl. Res. 2011, 57, 729–736. [Google Scholar] [CrossRef]
- Margalida, A.; Colomer, M.À.; Sánchez, R.; Sánchez, F.J.; Oria, J.; González, L.M. Behavioral evidence of hunting and foraging techniques by a top predator suggests the importance of scavenging for preadults. Ecol. Evol. 2017, 7, 4192–4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, J.E.; Zuberogoitia, I.; Gómez, G.; Escarabajal, J.M.; Cerezo, E.; Jiménez-Franco, M.V.; Calvo, J.F. Attack success in Bonelli’s Eagle Aquila fasciata. Ornis Fenn. 2014, 91, 67–78. [Google Scholar]
- Moleón, M.; Bautista, J.; Sánchez-Zapata, J.A.; Gil-Sánchez, J.M. Diet of non-breeding Bonelli’s EaglesHieraaetus fasciatusat settlement areas of southern Spain. Bird Study 2009, 56, 142–146. [Google Scholar] [CrossRef] [Green Version]
- Zhelev, P.; Gradev, G.; Ivanov, I.; Georgiev, D. On the food spectrum of juvenile Eastern Imperial Eagles (Aquila heliaca Savigny, 1809) in Southern Bulgaria. Ecol. Balk. 2009, 1, 51–58. (In Bulgarian) [Google Scholar]
- Morrison, J.L.; Wood, P.B. Broadening Our Approaches to Studying Dispersal in Raptors. J. Raptor Res. 2009, 43, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Soutullo, A.; López-López, P.; Urios, V. Incorporating spatial structure and stochasticity in endangered Bonelli’s eagle’s population models: Implications for conservation and management. Biol. Conserv. 2008, 141, 1013–1020. [Google Scholar] [CrossRef] [Green Version]
- Balbontín, J. Identifying suitable habitat for dispersal in Bonelli’s eagle: An important issue in halting its decline in Europe. Biol. Conserv. 2005, 126, 74–83. [Google Scholar] [CrossRef]
- Ferrer, M. The Spanish Imperial Eagle; Lynx Edicions: Barcelona, Spain, 2001; 224p. [Google Scholar]
- Demerdzhiev, D. Eastern Imperial Eagle (Aquila heliaca heliaca Savigny, 1809) (Accipitridae—Aves) in Bulgaria—Distribution, Biology, Ecology, Numbers, and Conservation Measures. Synopsis of the Ph.D. Thesis, BAS-NMNH, Sofia, Bulgaria, 2011. (In Bulgarian, with English Summary). [Google Scholar]
- Zuluaga, S.; Vargas, F.H.; Aráoz, R.; Grande, J.M. Main aerial top predator of the Andean Montane Forest copes with fragmentation, but may be paying a high cost. Glob. Ecol. Conserv. 2022, 37, e02174. [Google Scholar] [CrossRef]
- Demerdzhiev, D.A.; Horváth, M.; Kovács, A.; Stoychev, S.A.; Karyakin, I.V. Status and population trend of the eastern imperial eagle (Aquila heliaca) in Europe in the period 2000–2010. Acta Zool. Bulg. 2011, 3, 5–14. [Google Scholar]
- Ferguson-Lees, J.; Christie, D.A. Raptors of the World; Christopher Helm: London, UK, 2001. [Google Scholar]
- Abuladze, A. Ecology of the Imperial Eagle Aquila heliaca in Georgia. In Eagle Studies; Meyburg, B.-U., Chancellor, R.D., Eds.; WWGBP: Berlin, Germany; Paris, France; London, UK, 1996; pp. 447–457. [Google Scholar]
- Chavko, J.; Danko, Š.; Obuch, J.; Mihók, J. The Food of the Imperial Eagle (Aquila heliaca) in Slovakia. Slov. Rap. J. 2007, 1, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Demerdzhiev, D.; Dobrev, D.; Stoychev, S.; Terziev, N.; Spasov, S.; Boev, Z. Distribution, abundance, breeding parameters, threats and prey preferences of the eastern imperial eagle (Aquila heliaca) in European Turkey. Slovak Raptor J. 2014, 8, 17–25. [Google Scholar] [CrossRef]
- Horváth, M.; Solti, B.; Fatér, I.; Juhász, T.; Haraszthy, L.; Szitta, T.; Ballók, Z.; Pásztory-Kovács, S. Temporal changes in the diet composition of the Eastern Imperial Eagle (Aquila heliaca) in Hungary. Ornis Hung. 2018, 26, 1–26. [Google Scholar] [CrossRef]
- Karyakin, I.; Kovalenko, A.; Levin, A.; Pazhenkov, A. Eagles of the Aral—Caspian Region, Kazakhstan. Rap. Con. 2011, 22, 92–152. [Google Scholar]
- Karyakin, I.; Nikolenko, E.; Levin, A.; Kovalenko, A. Imperial Eagle in Russia and Kazakhstan: Population status and trends. Rap. Con. 2008, 14, 18–27. [Google Scholar]
- Katzner, T.; Bragin, E.; Knick, S.; Smith, A. Spatial structure in the diet of Eastern Imperial Eagle Aquila heliaca in Kazakhstan. J. Avian Biol. 2006, 37, 594–600. [Google Scholar] [CrossRef]
- BirdLife International. Species Factsheet: Aquila heliaca. Available online: http://www.birdlife.org (accessed on 24 October 2020).
- Demerdzhiev, D.; Dobrev, D.; Popgeorgiev, G.; Stoychev, S. Landscape alteration affects the demography of an endangered avian predator by reducing the habitat quality. Avian Res. 2022, 13, 100030. [Google Scholar] [CrossRef]
- Lazarova, I.; Dobrev, D.; Gradev, G.; Petrov, R.; Stoychev, S.; Klisurov, I.; Demerdzhiev, D. Main mortality factors for the Eastern Imperial Eagle (Aquila heliaca Savigny, 1809) in Bulgaria. Orn. Hung. 2020, 28, 120–134. [Google Scholar] [CrossRef]
- Hadad, E.; Zduniak, P.; Yosef, R. Sustaining Increasing Wintering Raptor Populations in Central Israel: A 38 Years Perspective. Sustainability 2022, 14, 12481. [Google Scholar] [CrossRef]
- Angelov, I. Numbers, Ecology, Behavior, and Measures for the Conservation of the Imperial Eagle (Aquila heliaca) in the Vicinity of the Town of Sliven. Master’s thesis, Department of Zoology and Anthropology, Faculty of Biology, Sofia University, Tokyo, Japan, 2009; 82p. (In Bulgarian). [Google Scholar]
- Tzonev, R.; Gussev, C.; Popgeorgiev, G. Scrub and grassland habitats of Besaparski Ridove Special Protection Area (Natura 2000), southern Bulgaria: Distribution and assessment of their conservation status. Acta Zool. Bulg. 2014, 5, 137–142. [Google Scholar]
- Forsman, D. Eastern Imperial Eagle Plumages. Alula 2005, 4, 147–152. [Google Scholar]
- Cresswell, W. Flocking is an effective anti-predation strategy in red-shanks, Tringa totanus. Anim. Behav. 1994, 47, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Cresswell, W.; Quinn, J.L. Faced with a choice, sparrowhawks more attack the more vulnerable prey group. Oikos 2004, 104, 71–76. [Google Scholar] [CrossRef]
- Collopy, M.W. Foraging Behavior and Success of Golden Eagles. Ornithology 1983, 100, 747–749. [Google Scholar] [CrossRef]
- Watson, J. The Golden Eagle, 2nd ed.; Yale University Press: New Haven, CT, USA, 2010; 374p. [Google Scholar]
- Ellis, D.H.; Schmitt, N.J. Behavior of the Golden Eagle. An Illustrated ethogram; Hancock House Publishers: Surrey, BC, Canada, 2017; 104p. [Google Scholar]
- Gotelli, N.J.; Graves, G.R. Null Models in Ecology; Smithsonian Institution Press: Washington, DC, USA, 1996. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Chao, A.; Lee, S.M. Estimating the number of classes via sample coverage. J. Am. Stat. Assoc. 1992, 87, 210–217. [Google Scholar] [CrossRef]
- Chao, A.; Shen, T.J. Nonparametric estimation of Shannon’s diversity index when there are unseen species in sample. Environm. Ecolog. Stat. 2003, 10, 429–443. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95364-7. [Google Scholar]
- StatSoft Inc. STATISTICA (Data Analysis Software System), Version 12. StatSoft Inc.: Tulsa, OK, USA, 2013; Available online: www.statsoft.com (accessed on 9 September 2022).
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Paleont. Elec. 2001, 4, 9. [Google Scholar]
- Heath, J.A.; Kochert, M.N.; Steenhof, K. Golden Eagle dietary shifts following wildfire and shrub loss have negative consequences for nestling survivorship. Ornithol. Appl. 2021, 123, duab034. [Google Scholar] [CrossRef]
- Milchev, B. Diet shifting of tortoise-eating Golden Eagles (Aquila chrysaetos) in southeastern Bulgaria. Ornis Fenn. 2022, 99, 60–70. [Google Scholar] [CrossRef]
- Brockmann, H.; Barnard, C. Kleptoparasitism in birds. Anim. Behav. 1979, 27, 487–514. [Google Scholar] [CrossRef]
- Steele, W.; Hockey, P. Factors Influencing Rate and Success of Intraspecific Kleptoparasitism among Kelp Gulls (Larus dominicanus). Ornithology 1995, 112, 847–859. [Google Scholar] [CrossRef] [Green Version]
- Cresswell, W. Kleptoparasitism rates and aggressive interactions between raptors. In Raptors Worldwide: Proceedings of the 6th Conference on Birds of Prey and Owls; Chancellor, R.D., Meyburg, B.U., Eds.; World Working Group on Birds of Prey and Owls: Budapest, Hungary, 2004; pp. 806–813. [Google Scholar]
- Edwards, G.P. Predicting seasonal diet in the yellow-bellied marmot: Success and failure for the linear programming model. Oecologia 1997, 112, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Meyburg, B.U.; Scheller, W.; Bergmanis, U. Home range size, habitat utilization, hunting and time budgets of Lesser Spotted Eagles Aquila pomarina with regard to disturbance and landscape fragmentation. In Raptors Worldwide; Chancellor, R.D., Meyburg, B.U., Eds.; WWGBP & MME: Berlin, Germany, 2004. [Google Scholar]
- Mirski, P. Effect of selected environmental factors on hunting methods and hunting success in the lesser spotted eagle Aquila pomarina in North-Eastern Poland. Russ. J. Ecol. 2010, 41, 197–200. [Google Scholar] [CrossRef]
- Nadjafzadeh, M.; Hofer, H.; Krone, O. Sit-and-wait for large prey: Foraging strategy and prey choice of White-tailed Eagles. J. Ornithol. 2016, 157, 165–1178. [Google Scholar] [CrossRef]
- Kitowski, I. Age-related differences in foraging behavior of Montagu’s harrier Circus pygargus males in south-east Poland. Acta Ethol. 2003, 6, 35–38. [Google Scholar] [CrossRef]
- Rutz, C.; Whittingham, M.J.; Newton, I. Age-Dependent Diet Choice in an Avian Top Predator. Proc. Biol. Sci. 2019, 273, 579–586. Available online: https://www.jstor.org/stable/25223332 (accessed on 12 October 2020). [CrossRef]
- Marchetti, K.; Price, T. Differences in the Foraging of Juvenile and Adult Birds: The Importance of Developmental Constraints. Biol. Rev. 1989, 64, 51–70. [Google Scholar] [CrossRef]
- Koshev, Y.; Kachamakova, M.; Arangelov, S.; Ragyov, D. Translocations of European ground squirrel (Spermophilus citellus) along altitudinal gradient in Bulgaria—An overview. Nat. Conserv. 2006, 35, 63–95. [Google Scholar] [CrossRef]
- Demerdzhiev, D. Breeding parameters and factors infuencing the reproduction of an expanding Long-legged Buzzard (Buteo rufnus) population under high breeding density conditions. J. Ornithol. 2022, 163, 405–415. [Google Scholar] [CrossRef]
- Mañosa, S.; Real, J.; Codina, J. Selection of settlement areas by juvenile Bonelli’s eagle in Catalonia. J. Raptor. Res. 1988, 32, 208–214. [Google Scholar]
- Kachamakova, M.; Koshev, Y.; Rammou, D.-L.; Spasov, S. Rise and fall: Results of a multidisciplinary study and 5-year long monitoring of conservation translocation of the European ground squirrel. Biodivers. Data J. 2022, 10, e83321. [Google Scholar] [CrossRef]
- Demerdzhiev, D. Birds in Besaparski riodve special protection area (Natura 2000), Southern Bulgaria: Conservation status and dynamics. Acta Zool. Bulg. Suppl. 2014, 5, 171–189. [Google Scholar]





| Variable | Variable Type | Description |
|---|---|---|
| Age | Categorical | Three age-classes: adults, immature, and juveniles. |
| Attack/Foraging Mode | Categorical | Ten classes: (1) Powered contour flight; (2) High soar with vertical stoop and descent attack; (3) Glide attack with tail-chase; (4) High-perch; (5) Walk-grab; (6) Collect a crashed animal; (7) Kleptoparasitism; (8) Carrion feeding; (9) Cooperative feeding; (10) Unspecified |
| Prey type | Categorical | Prey species. The prey’s single specimens are grouped into a common category. Nonidentified prey was given in a separate category. |
| Habitat type | Categorical | Habitat characteristics according to land use pattern: (1) Pasture; (2) Stubble; (3) Wheat; (4) Fallow; (5) Other, including single casses such as asfalt road, ekoton, quarry, shrubs, and fishpond;.(6) The airstrikes were divided into a separate category: “in the air” |
| Vegetation height | Continuous | The height of the vegetation in cm. |
| Seasons | Categorical | Two categories with an equal duration: (1) Spring–summer (from March to August) and (2) autum–winter (from September to February) |
| N | Model Structure | AIC | ΔAIC | df | w | p |
|---|---|---|---|---|---|---|
| 1 | Seasons + Foraging Mode + Habitat type | 175.77 | 0.00 | 13 | 0.42 | <0.001 |
| 2 | Foraging Mode + Habitat type | 177.16 | 1.39 | 12 | 0.21 | <0.001 |
| 3 | Prey type + Habitat type | 177.26 | 1.50 | 20 | 0.20 | <0.001 |
| 4 | Seasons + Foraging Mode + Habitat type + Vegetation (cm) | 177.43 | 1.66 | 14 | 0.17 | <0.001 |
| N | Explanatory factors | β2 | St.err. | Lower CL/Upper CL | Wald Stat. | p |
| 1 | Spring–summer season | −0.67 | 0.34 | −1.34/0.00 | 3.82 | 0.051 |
| 2 | Kleptoparasitism | −4.35 | 1.77 | −7.81/−0.88 | 6.03 | 0.01 |
| 3 | European Souslik | −2.76 | 1.40 | −5.51/−0.01 | 3.87 | 0.049 |
| 4 | Rodents (excl. Souslik) | −4.52 | 1.78 | −8.02/−1.03 | 6.43 | 0.01 |
| 5 | Pasture | 2.96 | 0.54 | 1.90/4.02 | 30.07 | <0.001 |
| 6 | In the air | 4.16 | 0.80 | 2.60/5.72 | 27.29 | <0.001 |
| 7 | Other habitats | 4.17 | 1.05 | 2.12/6.22 | 15.87 | <0.001 |
| 8 | Wheat | 4.31 | 0.67 | 3.00/5.62 | 41.46 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demerdzhiev, D.; Angelov, I.; Dobrev, D. Foraging Patterns of Non-Territorial Eastern Imperial Eagle (Aquila heliaca): A Case of Successful Adaptation. Diversity 2022, 14, 1060. https://doi.org/10.3390/d14121060
Demerdzhiev D, Angelov I, Dobrev D. Foraging Patterns of Non-Territorial Eastern Imperial Eagle (Aquila heliaca): A Case of Successful Adaptation. Diversity. 2022; 14(12):1060. https://doi.org/10.3390/d14121060
Chicago/Turabian StyleDemerdzhiev, Dimitar, Ivaylo Angelov, and Dobromir Dobrev. 2022. "Foraging Patterns of Non-Territorial Eastern Imperial Eagle (Aquila heliaca): A Case of Successful Adaptation" Diversity 14, no. 12: 1060. https://doi.org/10.3390/d14121060
APA StyleDemerdzhiev, D., Angelov, I., & Dobrev, D. (2022). Foraging Patterns of Non-Territorial Eastern Imperial Eagle (Aquila heliaca): A Case of Successful Adaptation. Diversity, 14(12), 1060. https://doi.org/10.3390/d14121060