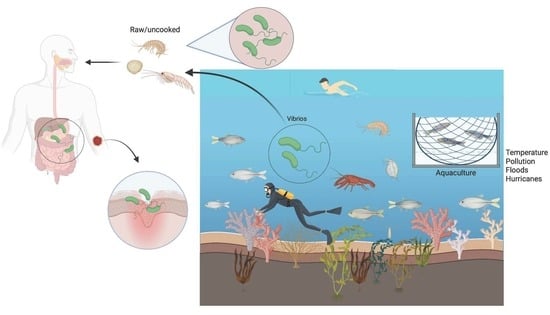
Vibrio spp.: Life Strategies, Ecology, and Risks in a Changing Environment
Abstract
:1. Introduction
2. Vibrio as an Heterotroph—Free Living Way of Life
3. Vibrio spp. Associated with Micro- and Macroalgae
4. Vibrio spp. Associated with Zooplankton and Animals
5. Symbiotic Members of Vibrionaceae
6. Vibrio spp. as Human Pathogens
7. Antibiotic Susceptibility in Vibrio spp.
8. Risks Related to Vibrio spp.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wright, A.C.; Hill, R.T.; Johnson, J.A.; Roghman, M.C.; Colwell, R.R.; Morris, J.G. Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl. Environ. Microbiol. 1996, 62, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.R.; Marcelino, L.A.; Polz, M.F. Diversity, sources and detection of human bacterial pathogens in the marine environment. In Oceans and Health: Pathogens in the Marine Environment; Belkin, S., Colwell, R.R., Eds.; Springer: New York, NY, USA, 2005; pp. 29–68. [Google Scholar] [CrossRef]
- Barbieri, E.; Falzano, L.; Fiorentini, C.; Pianetti, A.; Baffone, W.; Fabbri, A.; Matarrese, P.; Casiere, A.; Katouli, M.; Kuhn, I.; et al. Occurrence, diversity, and pathogenicity of halophilic Vibrio spp. and non-O1 Vibrio cholerae from estuarine waters along the Italian Adriatic coast. Appl. Environ. Microbiol. 1999, 65, 2748–2753. [Google Scholar] [CrossRef] [Green Version]
- Denner, E.B.M.; Vybiral, D.; Fischer, U.R.; Velimirov, B.; Busse, H.J. Vibrio calviensis sp. nov., a halophilic, facultatively oligotrophic 0.2 micron-filterable marine bacterium. Int. J. Syst. Evol. Microbiol. 2002, 52, 549–553. [Google Scholar] [CrossRef] [Green Version]
- Heidelberg, J.F.; Heidelberg, K.B.; Colwell, R.R. Seasonality of Chesapeake Bay bacterioplankton species. Appl. Environ. Microbiol. 2002, 68, 5488–5497. [Google Scholar] [CrossRef] [Green Version]
- Vezzulli, L.; Pezzati, E.; Moreno, M.; Fabiano, M.; Pane, L.; Pruzzo, C.; VibrioSea Consortium. Benthic ecology of Vibrio spp. and pathogenic Vibrio species in a coastal Mediterranean environment (La Spezia Gulf, Italy). Microb. Ecol. 2009, 58, 808–818. [Google Scholar] [CrossRef]
- Baumann, P.; Furniss, A.L.; Lee, J.V. Genus, I. Vibrio. In Bergey’s Manual of Systematic Bacteriology, 1st ed.; Krieg, N.R., Holt, J.G., Eds.; The Williams & Wilkins Co.: Baltimore, MA, USA, 1984; Volume 1, pp. 518–538. [Google Scholar]
- Genus Vibrio. Available online: www.bacterio.net/vibrio.html (accessed on 4 November 2021).
- Chakraborty, S.; Nair, G.B.; Shinoda, S. Pathogenic vibrios in the natural aquatic environment. Rev. Environ. Health 1997, 12, 63–80. [Google Scholar] [CrossRef]
- Pruzzo, C.; Huq, A.; Colwell, R.R.; Donelli, G. Pathogenic Vibrio species in the marine and estuarine environment. In Oceans and Health: Pathogens in the Marine Environment; Belkin, S., Colwell, R.R., Eds.; Springer: New York, NY, USA, 2005; pp. 217–252. [Google Scholar] [CrossRef]
- Nair, G.B.; Faruque, S.M.; Sack, D.A. 13-Vibrios. In Emerging Foodborne Pathogens; Motarjemi, Y., Adams, M., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2006; pp. 332–372. [Google Scholar] [CrossRef]
- Jones, J.L. Vibrio: Introduction, Including Vibrio parahaemolyticus, Vibrio vulnificus, and other Vibrio species. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: London, UK, 2014; pp. 691–698. [Google Scholar]
- Farmer, J.J., III; Michael Janda, J.; Brenner, F.W.; Cameron, D.N.; Birkhead, K.M. Vibrio . In Bergey’s Manual of Systematics of Archaea and Bacteria; The Editorial Board; In Association with Bergey’s Manual Trust; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–79. [Google Scholar]
- Pacini, F. Osservazione microscopiche e deduzioni patologiche sul Cholera Asiatico. Gaz Med. Ital. Toscana Firenze 1854, 6, 405–412. [Google Scholar]
- Bally, M.; Garrabou, J. Thermodependent bacterial pathogens and mass mortalities in temperate benthic communities: A new case of emerging disease linked to climate change. Glob. Chang. Biol. 2007, 13, 2078–2088. [Google Scholar] [CrossRef]
- Heidelberg, J.F.; Eisen, J.A.; Nelson, W.C.; Clayton, R.A.; Gwinn, M.L.; Dodson, R.J.; Haft, D.H.; Hickey, E.K.; Peterson, J.D.; Umayam, L.; et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholera. Nature 2000, 406, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Thompson, F.L.; Gevers, D.; Thompson, C.C.; Dawyndt, P.; Hoste, B.; Munn, C.B.; Swings, J. Phylogeny and molecular identification of vibrios on the basis of multilocus sequence analysis. Appl. Environ. Microbiol. 2005, 71, 5107–5115. [Google Scholar] [CrossRef] [Green Version]
- Sawabe, T.; Kita-Tsukamoto, K.; Thompson, F.L. Inferring the evolutionary history of vibrios by means of multilocus sequence analysis. J. Bacteriol. 2007, 189, 7932–7936. [Google Scholar] [CrossRef] [Green Version]
- Al-Saari, N.; Gao, F.; Rohul, A.A.K.M.; Sato, K.; Sato, K.; Mino, S.; Suda, W.; Oshima, K.; Hattori, M.; Ohkuma, M.; et al. Advanced microbial taxonomy combined with genome-based approaches reveals that Vibrio astriarenae sp. nov., an agarolytic marine bacterium, forms a new clade in Vibrionaceae. PLoS ONE 2015, 10, e0136279. [Google Scholar] [CrossRef] [Green Version]
- Farmer, J.J., III; Hickman-Brenner, F.W. The Genera Vibrio and Photobacterium. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 508–563. [Google Scholar] [CrossRef]
- Grimes, D.J. The vibrios: Scavengers, symbionts, and pathogens from the sea. Microb. Ecol. 2020, 80, 501–506. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Trinanes, J.; Martinez-Urtaza, J. The new tools revolutionizing Vibrio science. Environ. Microbiol. 2020, 22, 4096–4100. [Google Scholar] [CrossRef]
- Abioye, O.E.; Osunla, A.C.; Okoh, A.I. Molecular detection and distribution of six medically important Vibrio spp. in selected freshwater and brackish water resources in Eastern Cape Province, South Africa. Front. Microbiol. 2021, 12, 617703. [Google Scholar] [CrossRef]
- Percival, S.L.; Williams, D.W. Chapter Twelve—Vibrio. In Microbiology of Waterborne Diseases: Microbiological Aspects and Risks, 2nd ed.; Percival, S., Yates, M.V., Williams, D.W., Chalmers, R.M., Gray, N.F., Eds.; Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2014; pp. 237–248. [Google Scholar]
- McCarter, L. The multiple identities of Vibrio parahaemolyticus. J. Mol. Microbiol. Biotechnol. 1999, 1, 51–57. [Google Scholar]
- Lipp, E.K.; Huq, A.; Colwell, R.R. Effects of global climate on infectious disease: The cholera model. Clin. Microbiol. Rev. 2002, 15, 757–770. [Google Scholar] [CrossRef] [Green Version]
- Ruby, E.G. Lessons from a cooperative, bacterial-animal association: The Vibrio fischeri-Euprymna scolopes light organ symbiosis. Annu. Rev. Microbiol. 1996, 50, 591–624. [Google Scholar] [CrossRef] [Green Version]
- Grau, B.L.; Henk, M.C.; Pettis, G.S. High-frequency phase variation of Vibrio vulnificus 1003: Isolation and characterization of a rugose phenotypic variant. J. Bacteriol. 2005, 187, 2519–2525. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Bonnin-Jusserand, M.; Copin, S.; Le Bris, C.; Brauge, T.; Gay, M.; Brisabois, A.; Grard, T.; Midelet-Bourdin, G. Vibrio species involved in seafood-borne outbreaks (Vibrio cholerae, V. parahaemolyticus and V. vulnificus): Review of microbiological versus recent molecular detection methods in seafood products. Crit. Rev. Food Sci. Nutr. 2019, 59, 597–610. [Google Scholar] [CrossRef]
- Thompson, J.R.; Polz, M.F. Dynamics of Vibrio populations and their role in environmental nutrient cycling. In The Biology of Vibrios; Thompson, F.L., Austin, B., Swings, J., Eds.; American Society for Microbiology: Washington, DC, USA, 2006; pp. 190–203. [Google Scholar]
- Cottrell, M.T.; Kirchman, D.L. Contribution of major bacterial groups to bacterial biomass production (thymidine and leucine incorporation) in the Delaware estuary. Limnol. Oceanogr. 2003, 48, 168–178. [Google Scholar] [CrossRef]
- Beardsley, C.; Pernthaler, J.; Wosniok, W.; Amann, R. Are readily culturable bacteria in coastal North Sea waters suppressed by selective grazing mortality? Appl. Environ. Microbiol. 2003, 69, 2624–2630. [Google Scholar] [CrossRef] [Green Version]
- Oberbeckmann, S.; Fuchs, B.M.; Meiners, M.; Wichels, A.; Wiltshire, K.H.; Gerdts, G. Seasonal dynamics and modeling of a Vibrio community in coastal waters of the North Sea. Microb. Ecol. 2012, 63, 543–551. [Google Scholar] [CrossRef]
- Böer, S.I.; Heinemeyer, E.A.; Luden, K.; Erler, R.; Gerdts, G.; Janssen, F.; Brennholt, N. Temporal and spatial distribution patterns of potentially pathogenic Vibrio spp. at recreational beaches of the German North Sea. Microb. Ecol. 2013, 65, 1052–1067. [Google Scholar] [CrossRef] [Green Version]
- Rivera, I.N.G.; Souza, K.M.C.; Souza, C.P.; Lopes, R.M. Free-living and plankton-associated vibrios: Assessment in ballast water, harbor areas, and coastal ecosystems in Brazil. Front. Microbiol. 2013, 3, 443. [Google Scholar] [CrossRef] [Green Version]
- Di, D.Y.W.; Lee, A.; Jang, J.; Han, D.; Hura, H.-G. Season-specific occurrence of potentially pathogenic Vibrio spp. on the Southern Coast of South Korea. Appl. Environ. Microbiol. 2017, 83, e02680-16. [Google Scholar] [CrossRef] [Green Version]
- Gyraite, G.; Kataržytè, M.; Overlingè, D.; Vaičiūtè, D.; Jonikaitè, E.; Schernewski, G. Skip the dip—Avoid the risk? Integrated microbiological water quality assessment in the South-Eastern Baltic Sea coastal waters. Water 2020, 12, 3146. [Google Scholar] [CrossRef]
- Amirmozafari, N.; Forohesh, H.; Halakoo, A. Occurrence of pathogenic vibrios in coastal areas of Golestan Province in Iran. Arch. Razi Ins. 2005, 60, 33–44. [Google Scholar]
- Machado, A.; Bordalo, A.A. Detection and quantification of Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus in coastal waters of Guinea-Bissau (West Africa). EcoHealth 2016, 13, 339–349. [Google Scholar] [CrossRef]
- Costa, R.A.; Silva, G.C.; Peixoto, J.R.O.; Vieira, G.H.F.; Vieira, R.H.S.F. Quantification and distribution of Vibrio species in water from an estuary in Ceará-Brazil impacted by shrimp farming. Braz. J. Oceanogr. 2010, 8, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, D.I.; Moore, J.G.; Stewart, J.R.; Hilborn, E.D.; George, B.J.; Li, Q.; Dickerson, J.; Keppler, C.K.; Sandifer, P.A. Temporal and environmental factors driving Vibrio vulnificus and V. parahaemolyticus populations and their associations with harmful algal blooms in South Carolina detention ponds and receiving tidal creeks. Geohealth 2017, 1, 306–317. [Google Scholar] [CrossRef]
- Jesser, K.J.; Noble, R.T. Vibrio ecology in the Neuse River Estuary, North Carolina, characterized by Next-Generation Amplicon Sequencing of the gene encoding Heat Shock Protein 60 (hsp60). Appl. Environ. Microbiol. 2018, 84, e00333-18. [Google Scholar] [CrossRef] [Green Version]
- Kokashvili, T.; Whitehouse, C.A.; Tskhvediani, A.; Grim, C.J.; Elbakidze, T.; Mitaishvili, N.; Janelidze, N.; Jaiani, E.; Haley, B.J.; Lashkhi, N.; et al. Occurrence and diversity of clinically important Vibrio species in the aquatic environment of Georgia. Front. Public Health 2015, 3, 232. [Google Scholar] [CrossRef]
- Lipp, E.K.; Rodriguez-Palacios, C.; Rose, J.B. Occurrence and distribution of the human pathogen Vibrio vulnificus in a subtropical Gulf of Mexico estuary. Hydrobiologia 2001, 460, 165–173. [Google Scholar] [CrossRef]
- Griffitt, K.J.; Grimes, D.J. Abundance and distribution of Vibrio cholerae, V. parahaemolyticus, and V. vulnificus following a major freshwater intrusion into the Mississippi Sound. Microbial Ecol. 2013, 65, 578–583. [Google Scholar] [CrossRef]
- Sobrinho, P.S.C.; Destro, M.T.; Franco, B.D.G.M.; Landgraf, M. Correlation between environmental factors and prevalence of Vibrio parahaemolyticus in oysters harvested in the southern coastal area of Sao Paulo state, Brazil. Appl. Environ. Microbiol. 2010, 76, 1290–1293. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, W.B.; Paranjpye, R.N.; Hamel, O.S.; Hard, C.; Strom, M.S. Vibrio parahaemolyticus risk assessment in the Pacific Northwest: It’s not what’s in the water. FEMS Microbiol. Ecol. 2019, 95, fiz027. [Google Scholar] [CrossRef] [Green Version]
- La Rosa, T.; Mirto, S.; Mazzola, A.; Danovaro, R. Differential responses of benthic microbes and meiofauna to fish-farm disturbance in coastal sediments. Environ. Pollut. 2001, 112, 427–434. [Google Scholar] [CrossRef]
- Cabral, J.P.S. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef]
- Dufour, A.; Bartram, J.; Bos, R.; Gannon, V. Animal waste, water quality and human health. Water Intell Online 2013, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Delahoy, M.J.; Wodnik, B.; McAliley, L.; Penakalapati, G.; Swarthout, J.; Freeman, M.C.; Levy, K. Pathogens transmitted in animal feces in low- and middle-income countries. Int. J. Hyg. Environ. Health 2018, 221, 661–676. [Google Scholar] [CrossRef]
- Gardade, L.; Khandeparker, L. Spatio-temporal variations in pathogenic bacteria in the surface sediments of the Zuari estuary, Goa, India. Curr. Sci. 2017, 113, 1729–1738. [Google Scholar] [CrossRef]
- Shibata, T.; Solo-Gabriele, H.M.; Fleming, L.E.; Elmir, S. Monitoring marine recreational water quality using multiple microbial indicators in an urban tropical environment. Water Res. 2004, 38, 3119–3131. [Google Scholar] [CrossRef] [Green Version]
- Ghinsberg, R.C.; Drasinover, V.; Sheinberg, Y.; Nitzan, Y. Seasonal distribution of Aeromonas hydrophila and Vibrio species in Mediterranean coastal water and beaches: A possible health hazard. Biomed. Lett. 1995, 51, 151–159. [Google Scholar]
- Elmanama, A.A.; Fahd, M.I.; Afifi, S.; Abdallah, S.; Bahr, S. Microbiological beach sand quality in Gaza Strip in comparison to seawater quality. Environ. Res. 2005, 99, 1–10. [Google Scholar] [CrossRef]
- Oyelade, A.; Fagade, O.; Sanuth, H.; Anjolaiya, L.; Nwadike, B. Microbiological quality of some recreational beaches along the shoreline of Lagos State, Nigeria. J. Environ. Earth Sci. 2018, 8, 61–76. [Google Scholar]
- McDougald, D.; Kjelleberg, S. Adaptive responses of Vibrios. In The Biology of Vibrios; Thompson, F.L., Austin, B., Swings, J., Eds.; American Society for Microbiology: Washington, DC, USA, 2006; pp. 133–155. [Google Scholar]
- Rehnstam-Holm, A.S.; Godhe, A.; Härnström, K.; Raghunath, P.; Saravanan, V.; Collin, B.; Karunasagar, I. Association between phytoplankton and Vibrio spp. along the southwest coast of India: A mesocosm experiment. Aquat. Microb. Ecol. 2010, 58, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, J.L.; Fries, J.S.; Noble, R.T. Vibrio and phytoplankton dynamics during the summer of 2004 in a eutrophying estuary. Ecol. Appl. 2007, 17, S102–S109. [Google Scholar] [CrossRef]
- Karl, D.M. Microbial oceanography: Paradigms, processes and promise. Nat. Rev. Microbiol. 2007, 5, 759–769. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V.; Li, X.; Huang, H. Chapter 2—Marine Plants of Coral Reefs. In Coral Reef Marine Plants of Hainan Island; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2017; pp. 5–39. [Google Scholar]
- Barberi, O.N.; Byron, C.J.; Burkholder, K.M.; Gelais, A.T.S.; Williams, A.K. Assessment of bacterial pathogens on edible macroalgae in coastal waters. J. Appl. Phycol. 2020, 32, 683–696. [Google Scholar] [CrossRef] [Green Version]
- Carli, A.; Pane, L.; Casareto, L.; Bertone, S.; Pruzzo, C. Occurrence of Vibrio alginolyticus in Ligurian coast rock pools (Tyrrhenian Sea, Italy) and its association with the copepod Tigriopus fulvus (Fisher 1860). Appl. Environ. Microbiol. 1993, 59, 1960–1962. [Google Scholar] [CrossRef] [Green Version]
- Lipp, E.K.; Rivera, I.N.G.; Gil, A.I.; Espeland, E.M.; Choopun, N.; Louis, V.R.; Russek-Cohen, E.; Huq, A.; Colwell, R.R. Direct detection of Vibrio cholerae and ctxA in Peruvian coastal water and plankton by PCR. Appl. Environ. Microbiol. 2003, 69, 3676–3680. [Google Scholar] [CrossRef] [Green Version]
- Wai, S.N.; Mizunoe, Y.; Yoshida, S. How Vibrio cholerae survive during starvation. FEMS Microbiol. Lett. 1999, 180, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Cottrell, M.T.; Wood, D.N.; Yu, L.; Kirchman, D.L. Selected chitinase genes in cultured and uncultured marine bacteria in the alpha and gamma-subclasses of the proteobacteria. Appl. Environ. Microbiol. 2000, 66, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Riemann, L.; Azam, F. Widespread N-acetyl-D-glucosamine uptake among pelagic marine bacteria and its ecological implications. Appl. Environ. Microbiol. 2002, 68, 5554–5562. [Google Scholar] [CrossRef] [Green Version]
- Pruzzo, C.; Vezzulli, L.; Colwell, R.R. Global impact of Vibrio cholerae interactions with chitin. Environ. Microbiol. 2008, 10, 1400–1410. [Google Scholar] [CrossRef]
- Keyhani, N.O.; Roseman, S. Physiological aspects of chitin catabolism in marine bacteria. Biochim. Biophys. Acta 1999, 1473, 108–122. [Google Scholar] [CrossRef]
- Meibom, K.L.; Blokesch, M.; Dolganov, N.A.; Wu, C.Y.; Schoolnik, G.K. Chitin induces natural competence in Vibrio cholerae. Science 2005, 310, 1824–1827. [Google Scholar] [CrossRef]
- Blokesch, M. The lifestyle of Vibrio cholera fosters gene transfers. Microbe 2014, 9, 64–70. [Google Scholar]
- Sawabe, T.; Setogushi, N.; Inoue, S.; Tanaka, R.; Ootsubo, M.; Yoshimizu, M.; Ezura, Y. Acetic acid production of Vibrio halioticoli from alginate: A possible role for establishment of abalone-V. halioticoli association. Aquaculture 2003, 219, 671–679. [Google Scholar] [CrossRef]
- Zampieri, A.; Babbucci, M.; Carraro, L.; Milan, M.; Fasolato, L.; Cardazzo, B. Combining Culture-dependent and culture-independent methods: New methodology insight on the Vibrio community of Ruditapes philippinarum. Foods 2021, 10, 1271. [Google Scholar] [CrossRef]
- Grimes, D.J.; Stemmler, J.; Hada, H.; May, E.B.; Maneval, D.; Hetrick, F.M.; Jones, R.T.; Stoskopf, M.; Colwell, R.R. Vibrio species associated with mortality of sharks held in captivity. Microb. Ecol. 1984, 10, 271–282. [Google Scholar] [CrossRef]
- Ringo, E.; Birkbeck, T.H. Intestinal microflora of fish larvae and fry. Aquacult. Res. 1999, 30, 73–93. [Google Scholar] [CrossRef]
- Rosenberg, E.; Ben-Haim, Y. Microbial diseases of corals and global warming. Environ. Microbiol. 2002, 4, 318–326. [Google Scholar] [CrossRef]
- Vandenberghe, J.; Thompson, F.L.; Gomez-Gil, B.; Swings, J. Phenotypic diversity amongst Vibrio isolates from marine aquaculture systems. Aquaculture 2003, 219, 9–20. [Google Scholar] [CrossRef]
- Laviad-Shitrit, S.; Izhaki, I.; Halpern, M. Accumulating evidence suggests that some waterbird species are potential vectors of Vibrio cholerae. PLoS Pathog. 2019, 15, e1007814. [Google Scholar] [CrossRef]
- Passalacqua, P.L.; Zavatta, E.; Bignami, G.; Serraino, A.; Serratore, P.; Studiorum, A.M.; Emilia, O. Occurrence of Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus in the clam Ruditapes philippinarum (Adams & Reeve, 1850) from Emilia Romagna and Sardinia, Italy. Ital. J. Food Saf. 2016, 5, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Romero, A.; Costa, M.D.; Forn-Cuni, G.; Balseiro, P.; Chamorro, R.; Dios, S.; Figueras, A.; Novoa, B. Occurrence, seasonality and infectivity of Vibrio strains in natural populations of mussels Mytilus galloprovincialis. Dis. Aquat. Org. 2014, 108, 149–163. [Google Scholar] [CrossRef]
- Lattos, A.; Bitchava, K.; Giantsis, A.I.; Theodorou, J.A.; Batargias, C.; Michaelidis, B. The implication of Vibrio bacteria in the winter mortalities of the critically endangered Pinna nobilis. Microorganisms 2021, 9, 922. [Google Scholar] [CrossRef]
- Brown, B.; Dunne, R.; Goodson, M.; Douglas, A. Experience shapes the susceptibility of a reef coral to bleaching. Coral Reefs 2002, 21, 19–126. [Google Scholar] [CrossRef]
- Richardson, L.L.; Goldberg, W.M.; Kuta, K.G. Florida’s mystery coral-killer identified. Nature 1998, 392, 557–558. [Google Scholar] [CrossRef]
- Sutherland, K.P.; Porter, J.W.; Torres, C. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 2004, 266, 273–302. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, E.; Falkovitz, L. The Vibrio shiloi/Oculina patagonica model system of coral bleaching. Ann. Rev. Microbiol. 2004, 58, 143–159. [Google Scholar] [CrossRef]
- Banin, E.; Israel, Y.; Kushmaro, T.; Loya, A.; Orr, Y.; Rosenberg, E. Penetration of the coral-bleaching bacterium Vibrio shiloi into Oculina patagonica. Appl. Environ. Microbiol. 2000, 66, 3031–3036. [Google Scholar] [CrossRef] [Green Version]
- Ben-Haim, Y.; Rosenberg, E. A novel Vibrio sp. pathogen of the coral Pocillopora damicornis. Mar. Biol. 2002, 141, 47–55. [Google Scholar]
- Hoffmann, M.; Fischer, M.; Ottesen, A.; McCarthy, P.J.; Lopez, J.V.; Brown, E.W.; Monday, S.R. Population dynamics of Vibrio spp. associated with marine sponge microcosms. ISME J. 2010, 4, 1608–1612. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, J.R.; Walder, J.D.; Colwell, R.R. Deep-sea bacteria: Growth and utilization of hydrocarbons at ambient and in situ pressure. Appl. Microbiol. 1974, 28, 982–986. [Google Scholar] [CrossRef]
- Raguénès, G.; Christen, R.; Guezennec, J.; Pignet, P.; Barbier, G. Vibrio diabolicus sp. nov., a new polysaccharide-secreting organism isolated from a deep-sea hydrothermal vent Polychaete annelid, Alvinella pompejana. Int. J. Syst. Evol. Microbiol. 1997, 47, 989–995. [Google Scholar] [CrossRef] [Green Version]
- Hasan, N.A.; Grim, C.J.; Lipp, E.K.; Rivera, I.N.; Chun, J.; Haley, B.J.; Taviani, E.; Choi, S.Y.; Hoq, M.; Munk, A.C.; et al. Deep-sea hydrothermal vent bacteria related to human pathogenic Vibrio species. Proc. Natl. Acad. Sci. USA 2015, 112, E2813–E2819. [Google Scholar] [CrossRef] [Green Version]
- Lightner, D.V.; Redman, R.M. Shrimp diseases and current diagnostic methods. Aquaculture 1998, 164, 201–220. [Google Scholar] [CrossRef]
- Leano, E.M.; Lavilla-Pitogo, C.R.; Paner, M.G. Bacterial flora in the hepatopancreas of pond-reared Penaeus monodon juveniles with luminous vibriosis. Aquaculture 1998, 164, 367–374. [Google Scholar] [CrossRef]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish, 3rd ed.; Springer-Verlag KG: Berlin, Germany, 1999; pp. 499–601. [Google Scholar]
- Borrego, J.J.; Castro, D.; Luque, A.; Paillard, C.; Maes, P.; Garcia, M.T.; Ventosa, A. Vibrio tapetis sp. nov., the causative agent of the brown ring disease affecting cultured clams. Int. J. Syst. Bacteriol. 1996, 46, 480–484. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.K.; Yu, S.R.; Chen, F.R.; Yang, T.I.; Liu, P.C. Virulence of Vibrio alginolyticus isolated from diseased tiger prawn, Penaeus monodon. Curr. Microbiol. 1996, 32, 229–231. [Google Scholar] [CrossRef]
- Lambert, C.; Nicolas, J.L.; Cilia, V.; Corre, S. Vibrio pectenicida sp. nov., a pathogen of scallop (Pecten maximus) larvae. Int. J. Syst. Bacteriol. 1998, 48, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Diggles, B.K.; Carson, J.; Hine, P.M.; Hickman, R.W.; Tait, M.J. Vibrio species associated with mortalities in hatchery-reared turbot (Colistium nudipinnis) and brill (C. guntheri) in New Zealand. Aquaculture 2000, 183, 1–12. [Google Scholar] [CrossRef]
- Olafsen, J.A. Interactions between fish larvae and bacteria in marine aquaculture. Aquaculture 2001, 200, 223–247. [Google Scholar] [CrossRef]
- Mechri, B.; Medhioub, A.; Medhioub, M.N.; Aouni, M. Diversity of Vibrionaceae associated with Ruditapes decussatus hatchery in Tunisia. Ann. Microbiol. 2012, 62, 597–606. [Google Scholar] [CrossRef]
- Stabili, L.; Di Salvo, M.; Alifano, P.; Tala, A. An integrative, multiparametric approach for the comprehensive assessment of microbial quality and pollution in aquaculture systems. Microb. Ecol. 2021. [Google Scholar] [CrossRef]
- Urbanczyk, H.; Ast, J.C.; Higgins, M.J.; Carson, J.; Dunlap, P.V. Reclassification of Vibrio fischeri, Vibrio logei, Vibrio salmonicida and Vibrio wodanis as Aliivibrio fischeri gen. nov., comb. nov., Aliivibrio logei comb. nov., Aliivibrio salmonicida comb. nov. and Aliivibrio wodanis comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 2823–2829. [Google Scholar] [CrossRef] [Green Version]
- Urbanczyk, H.; Ast, J.C.; Dunlap, P.V. Phylogeny, genomics, and symbiosis of Photobacterium. FEMS Microbiol. Rev. 2011, 35, 324–342. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, P.V.; Nakamura, M. Functional morphology of the luminescence system of Siphamia versicolor (Perciformes: Apogonidae), a bacterially luminous coral reef fish. J. Morphol. 2011, 272, 897–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlap, P.V.; Davis, K.M.; Tomiyama, S.; Fujino, M.; Fukui, A. Developmental and microbiological analysis of the inception of bioluminescent symbiosis in the marine fish Nuchequula nuchalis (Perciformes: Leiognathidae). Appl. Environ. Microbiol. 2008, 74, 7471–7481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ast, J.C.; Cleenwerck, I.; Engelbeen, K.; Urbanczyk, H.; Thompson, F.L.; De Vos, P.; Dunlap, P.V. Photobacterium kishitanii sp. nov., a luminous marine bacterium symbiotic with deep-sea fishes. Int. J. Syst. Evol. Microbiol. 2007, 57, 2073–2078. [Google Scholar] [CrossRef]
- McFall-Ngai, M.J.; Morin, J.G. Camouflage by disruptive illumination in leiognathids, a family of shallow water bioluminescent fishes. J. Exp. Biol. 1991, 156, 119–137. [Google Scholar] [CrossRef]
- Woodland, D.J.; Cabanban, A.S.; Taylor, V.M.; Taylor, R.J. A synchronized rhythmic flashing light display by schooling Leiognathus splendens (Leiognathidae: Perciformes). Mar. Freshw. Res. 2002, 53, 159–162. [Google Scholar] [CrossRef]
- Kimura, S.; Dunlap, P.V.; Peristiwady, T.; Lavilla-Pitogo, C.R. The Leiognathus aureus complex (Perciformes: Leiognathidae) with the description of a new species. Ichthyol. Res. 2003, 50, 221–232. [Google Scholar] [CrossRef]
- Wada, M.; Azuma, N.; Mizuno, N.; Kurokura, H. Transfer of symbiotic luminous bacteria from parental Leiognathus nuchalis to offspring. Mar. Biol. 1999, 135, 683–687. [Google Scholar] [CrossRef]
- Haygood, M.G.; Tebo, B.M.; Nealson, K.H. Luminous bacteria of a monocentrid fish (Monocentris japonicus) and two anomalopid fish (Photoblepharon palpebratus and Kryptophaneron alfredi): Population sizes and growth within the light organs, and rates of release into the seawater. Mar. Biol. 1984, 78, 249–254. [Google Scholar] [CrossRef]
- Gould, A.L.; Fritts-Penniman, A.; Gaisiner, A. Museum genomics illuminate the high specificity of a bioluminescent symbiosis for a genus of reef fish. Front. Ecol. Evol. 2021, 9, 630207. [Google Scholar] [CrossRef]
- Herring, P.J.; Clarke, M.R.; von Boletzky, S.; Ryan, K.P. The light organs of Sepiola atlantica and Spirula spirula (Mollusca: Cephalopoda): Bacterial and intrinsic systems in the order Sepioidea. J. Mar. Biol. Assoc. 1981, 61, 901–916. [Google Scholar] [CrossRef]
- Fidopiastis, P.M.; von Boletzky, S.; Ruby, E.G. A New Niche for Vibrio logei, the predominant light organ symbiont of squids in the genus Sepiola. J. Bacteriol. 1998, 180, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiguchi, M.K. Temperature affects species distribution in symbiotic populations of Vibrio spp. Appl. Env. Microbiol. 2000, 66, 3550–3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tischler, A.H.; Hodge-Hanson, K.M.; Visick, K.L. Vibrio fischeri–Squid Symbiosis; John Wiley & Sons, Ltd.: Chichester, UK, 2019. [Google Scholar] [CrossRef]
- Nyholm, S.V.; Stabb, E.V.; Ruby, E.G.; McFall-Ngai, M.J. Establishment of an animal-bacterial association: Recruiting symbiotic vibrios from the environment. Proc. Natl. Acad. Sci. USA 2000, 97, 10231–10235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norsworthy, A.N.; Visick, K.L. Gimme shelter: How Vibrio fischeri successfully navigates an animal’s multiple environments. Front. Microbiol. 2013, 4, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altura, M.A.; Heath-Heckman, E.A.C.; Gillette, A.; Kremer, N.; Krachler, A.M.; Brennan, C.; Ruby, E.G.; Orth, K.; McFall-Ngai, M.J. The first engagement of partners in the Euprymna scolopes-Vibrio fischeri symbiosis is a two-step process initiated by a few environmental symbiont cells. Environ. Microbiol. 2013, 15, 2937–2950. [Google Scholar] [CrossRef] [Green Version]
- Nyholm, S.V.; McFall-Ngai, M.J. The winnowing: Establishing the squid-Vibrio symbiosis. Nat. Rev. Microbiol. 2004, 2, 632–642. [Google Scholar] [CrossRef]
- Aschtgen, M.S.; Brennan, C.A.; Nikolakakis, K.; Cohen, S.; McFall-Ngai, M.; Ruby, E.G. Insights into flagellar function and mechanism from the squid–vibrio symbiosis. NPJ Biofilms Microbiomes 2019, 5, 32. [Google Scholar] [CrossRef]
- Millikan, D.S.; Ruby, E.G. Alterations in Vibrio fischeri motility correlate with a delay in symbiosis initiation and are associated with additional symbiotic colonization defects. Appl. Environ. Microbiol. 2002, 68, 2519–2528. [Google Scholar] [CrossRef] [Green Version]
- Wier, A.M.; Nyholm, S.V.; Mandel, M.J.; Massengo-Tiassé, R.P.; Schaefer, A.L.; Koroleva, I.; Splinter-BonDurant, S.; Brown, B.; Manzella, L.; Snir, E.; et al. Transcriptional patterns in both host and bacterium underlie a daily rhythm of anatomical and metabolic change in a beneficial symbiosis. Proc. Natl. Acad. Sci. USA 2010, 107, 2259–2264. [Google Scholar] [CrossRef] [Green Version]
- McFall-Ngai, M.J.; Ruby, E.G. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science 1991, 254, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.M.; Greenberg, E.P. Physical and functional maps of the luminescence gene cluster in an autoinducer-deficient Vibrio fischeri strain isolated from a squid light organ. J. Bacteriol. 1992, 174, 4384–4390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.C.; Miyashiro, T. Quorum sensing in the squid-Vibrio symbiosis Int. J. Mol. Sci. 2013, 14, 16386–16401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupp, C.; Urbanowski, M.; Greenberg, E.P.; Ruby, E.G. The Vibrio fischeri quorum-sensing systems ain and lux sequentially induce luminescence gene expression and are important for persistence in the squid host. Mol. Microbiol. 2003, 50, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Oliver, J.D.; Alamo, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- 130. CDC (Center for Disease Control and Prevention). Preliminary incidence and trends of infections with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2016–2019. MMWR 2020, 69, 509–514. [Google Scholar]
- Wachsmuth, I.K.; Blake, P.A.; Olsvik, O. Vibrio cholerae and cholera. In Molecular to Global Perspectives; ASM Press: Washington, DC, USA, 1994. [Google Scholar]
- Finkelstein, R.; Edelstein, S.; Mahamid, G. Fulminant wound infections due to Vibrio vulnificus. Isr. Med. Assoc. J. 2002, 4, 654–655. [Google Scholar] [PubMed]
- Singleton, F.L.; Attwell, R.; Jangi, S.; Colwell, R.R. Effects of temperature and salinity on Vibrio cholerae growth. Appl. Environ. Microbiol. 1982, 44, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- ECDC (European Centre for Disease Prevention and Control). Cholera. In ECDC. Annual Epidemiological Report for 2019; ECDC: Stockholm, Sweden, 2021. [Google Scholar]
- Ramamurthy, T.; Mutreja, A.; Weill, F.X.; Das, B.; Ghosh, A.; Nair, G.B. Revisiting the global epidemiology of cholera in conjunction with the genomics of Vibrio cholera. Front. Public Health 2019, 7, 237. [Google Scholar] [CrossRef]
- Islam, M.S.; Mahmuda, S.; Morshed, M.G.; Bakht, H.B.M.; Khan, M.N.H.; Sack, R.B.; Sack, D.A. Role of cyanobacteria in the persistence of Vibrio cholerae O139 in saline microcosms. Can. J. Microbiol. 2004, 50, 127–131. [Google Scholar] [CrossRef]
- Reidl, J.; Klose, K.E. Vibrio cholerae and cholera: Out of the water and into the host. FEMS Microbiol. Rev. 2002, 26, 125–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotter, P.A.; DiRita, V.J. Bacterial virulence gene regulation: An evolutionary perspective. Annu. Rev. Microbiol. 2000, 54, 519–565. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.R.; DiRita, V.J. Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol. Microbiol. 2002, 43, 119–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bina, J.; Zhu, J.; Dziejman, M.; Faruque, S.; Calderwood, S.; Mekalanos, J. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc. Natl. Acad. Sci. USA 2003, 100, 2801–2806. [Google Scholar] [CrossRef] [Green Version]
- Alter, T.; Appel, B.; Bartelt, E.; Dieckmann, R.; Eichhorn, C.; Erler, R.; Frank, C.; Gerdts, G.; Gunzer, F.; Hühn, S.; et al. Vibrio-Infektionen durch Lebensmittel und Meerwasser. Das Netzwerk “VibrioNet” stellt sich vor. Bundesgesundheitsblatt-Gesundh. -Gesundh. 2011, 54, 1235–1240. [Google Scholar] [CrossRef] [Green Version]
- Araj, G.F.; Taleb, R.; El Beayni, N.K.; Goksu, E. Vibrio albensis: An unusual urinary tract infection in a healthy male. J. Infect. Public Health 2019, 12, 712–713. [Google Scholar] [CrossRef]
- Fernández-Bravo, A.; Ballester, F.; Pujol, I.; Gomez-Bertomeu, F.; Martí, C.; Rezusta, A.; Ferrer-Cerón, I.; Aspiroz, C.; Puyod, M.J.; Figueras, M.J. Vibrio alginolyticus infections: Report of two cases from Spain with literature review. J. Med. Microb. Diagn. 2019, 8, 298. [Google Scholar] [CrossRef]
- Albuquerque, A.; Cardoso, H.; Pinheiro, D.; Macedo, G. Vibrio cholerae non-O1 and non-O139 bacteremia in a non-traveler Portuguese cirrhotic patient: First case report. Gastroenterol. Hepatol. 2013, 36, 309–310. [Google Scholar] [CrossRef]
- Montemayor, K.; Raju, S.; Sahetya, S.K. A fatal case of Vibrio cholerae bacteremia in a patient with stage IIIC ovarian carcinoma. American Thoracic Society International Conference Abstracts, C54. Critical Care Case Reports: Sepsis. Am. J. Respir. Crit. Care Med. 2016, 193, AS398. [Google Scholar]
- Kechker, P.; Senderovich, Y.; Ken-Dror, S.; Laviad-Shitrit, S.; Arakawa, E.; Halpern, M. Otitis media caused by V. cholerae O100: A case report and review of the literature. Front. Microbiol. 2017, 8, 1619. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-D.; Lai, L.-J.; Hsu, W.-H.; Huang, T.-Y. Vibrio cholerae non-O1—the first reported case of keratitis in a healthy patient. BMC Infect. Dis. 2019, 19, 916. [Google Scholar] [CrossRef] [PubMed]
- Brehm, T.T.; Berneking, L.; Rohde, H.; Chistner, M.; Schlickewei, C.; Martins, M.S.; Schmiedel, S. Wound infection with Vibrio harveyi following a traumatic leg amputation after a motorboat propeller injury in Mallorca, Spain: A case report and review of literature. BMC Infect. Dis. 2020, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Akram, A.; Stevens, R.P.; Konecny, P. Photobacterium damselae and Vibrio harveyi hand infection from marine exposure. Med. J. Aust. 2015, 203, 224–225.e1. [Google Scholar] [CrossRef]
- Jensen, J.; Jellinge, M.E. Severe septic shock and cardiac arrest in a patient with Vibrio metschnikovii: A case report. J. Med. Case Rep. 2014, 8, 348. [Google Scholar] [CrossRef] [Green Version]
- Konechnyi, Y.; Khorkavyi, Y.; Ivanchuk, K.; Kobza, I.; Sekowska, A.; Korniychuk, O. Vibrio metschnikovii: Current state of knowledge and discussion of recently identified clinical case. Clin. Case Rep. 2021, 9, 2236–2244. [Google Scholar] [CrossRef]
- Guillod, C.; Ghitti, F.; Mainetti, C. Vibrio parahaemolyticus induced cellulitis and septic shock after a sea beach holiday in a patient with leg ulcers. Case Rep. Dermatol. 2019, 11, 94–100. [Google Scholar] [CrossRef]
- França, J.C.B.; Raboni, S.M.; Sanfelice, E.; Polido, D.; Gentili, A.; Marques, F. Vibrio vulnificus infection in Southern Brazil—Case report. An. Bras. Dermatol. 2013, 88, 424–426. [Google Scholar] [CrossRef] [Green Version]
- Reitz, K.M.; Allen, P.D.; Hamad, G.G.; Corcos, A. A Case of Vibrio vulnificus septicemia in a patient with gastric bypass surgery. Surg. Infect. Case Rep. 2016, 1, 100–102. [Google Scholar] [CrossRef] [Green Version]
- Hendren, N.; Sukumar, S.; Glazer, C.S. Vibrio vulnificus septic shock due to a contaminated tattoo. BMJ Case Rep. 2017, 2017, bcr2017220199. [Google Scholar] [CrossRef]
- Guimarães, D.; Ribeiro, L.; Vieira, L.; Coelho, R. Necrotizing fasciitis caused by Photobacterium damselae: The first case in Portugal. Acta Med. Port. 2021, 34, 615–618. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration). Bad Bug Book: Foodborne Pathogenic Microorganisms and Natural Toxins Handbook, 2nd ed.; Lampel, K.A., Al-Khaldi, S., Cahill, S.M., Eds.; FDA: Washington, DC, USA, 2012.
- Jones, M.K.; Oliver, J.D. Vibrio vulnificus: Disease and pathogenesis. Infect. Immun. 2009, 77, 1723–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.H.; He, X.X.; Austin, B. Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Mar. Life Sci. Technol. 2020, 2, 231–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hundenborn, J.; Thurig, S.; Kommerell, M.; Haag, H.; Nolte, O. Severe wound infection with Photobacterium damselae ssp. damselae and Vibrio harveyi, following a laceration injury in marine environment: A case report and review of the literature. Case Rep. Med. 2013, 2013, 610632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC. Available online: https://www.cdc.gov/vibrio/index.html (accessed on 5 November 2021).
- Loo, K.Y.; Letchumanan, V.; Law, J.W.F.; Pusparajah, P.; Goh, B.H.; Mutalib, N.S.A.; He, Y.W.; Lee, L.H. Incidence of antibiotic resistance in Vibrio spp. Rev. Aquac. 2020, 12, 2590–2608. [Google Scholar] [CrossRef]
- Sack, D.A.; Lyke, C.; McLaughlin, C.; Suwanvanichkij, V. Antimicrobial Resistance in Shigellosis, Cholera and Campylobacteriosis; World Health Organization: Geneva, Switzerland, 2001; 56p. [Google Scholar]
- Dutta, D.; Kaushik, A.; Kumar, D.; Bag, S. Foodborne pathogenic vibrios: Antimicrobial resistance. Front. Microbiol. 2021, 2, 638331. [Google Scholar] [CrossRef]
- Das, B.; Verma, J.; Kumar, P.; Ghosh, A.; Ramamurthy, T. Antibiotic resistance in Vibrio cholerae: Understanding the ecology of resistance genes and mechanisms. Vaccine 2020, 38 (Suppl. 1), A83–A92. [Google Scholar] [CrossRef]
- Elmahdi, S.; DaSilva, L.V.; Parveen, S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: A review. Food Microbiol. 2016, 57, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Duque, A.; Gonzalez-Muñoz, A.; Arboleda-Valencia, J.; Vivas-Aguas, L.J.; Córdoba-Meza, T.; Rodriguez-Rey, G.T.; Díaz-Guevara, P.; Martinez-Urtaza, J.; Wiesner-Reyes, M. Comparative genomics of clinical and environmental isolates of Vibrio spp. of Colombia: Implications of traits associated with virulence and resistance. Pathogens 2021, 10, 1605. [Google Scholar] [CrossRef]
- Baker-Austin, C.; McArthur, J.V.; Tuckfield, R.C.; Najarro, M.; Lindell, A.H.; Gooch, J.; Stepanauskas, R. Antibiotic resistance in the shellfish pathogen Vibrio parahaemolyticus isolated from the coastal water and sediment of Georgia and South Carolina, USA. J. Food Prot. 2008, 71, 2552–2558. [Google Scholar] [CrossRef]
- Dobbs, F.C.; Goodrich, A.L.; Thomson, F.K.; Hynes, W. Pandemic serotypes of vibrio cholerae isolated from ships’ ballast tanks and coastal waters: Assessment of antibiotic resistance and virulence genes (tcpA and ctxA). Microb. Ecol. 2013, 65, 969–974. [Google Scholar] [CrossRef]
- Verma, J.; Bag, S.; Saha, B.; Kumar, P.; Ghosh, T.S.; Dayal, M.; Senapati, T.; Mehra, S.; Dey, P.; Desigamani, A.; et al. Genomic plasticity associated with antimicrobial resistance in Vibrio cholera. Proc. Natl. Acad. Sci. USA 2019, 116, 6226–6231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, K.S.; Goldstein, R.E.R.; He, X.; Jacobs, J.M.; Crump, B.C.; Sapkota, A.R. Antimicrobial susceptibility of Vibrio vulnificus and Vibrio parahaemolyticus recovered from recreational and commercial areas of Chesapeake Bay and Maryland Coastal Bays. PLoS ONE 2014, 9, e89616. [Google Scholar] [CrossRef] [PubMed]
- Gxalo, O.; Digban, T.O.; Igere, B.E.; Olapade, O.A.; Okoh, A.I.; Nwodo, U.U. Virulence and antibiotic resistance characteristics of Vibrio isolates from rustic environmental freshwaters. Front. Cel Front. Cell. Infect. Microbiol. 2021, 11, 732001. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, F.H.; Tschoeke, D.G.; Clementino, M.M.; Thompson, C.C.; Thompson, F.L. Genomic basis of antibiotic resistance in Vibrio parahaemolyticus strain JPA1. Mem. Inst. Oswaldo Cruz 2019, 114, e190053. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Q.; Xu, L.W.; Chen, H.X.; Liu, S.L.; Guo, Z.X.; Cheng, C.H.; Ma, H.L.; Feng, J. Prevalence, virulence genes, and antimicrobial resistance of Vibrio species isolated from diseased marine fish in South China. Sci. Rep. 2020, 10, 14329. [Google Scholar] [CrossRef] [PubMed]
- Amalina, N.Z.; Santha, S.; Zulperi, D.; Amal, M.N.A.; Yusof, M.T.; Zamri-Saad, M.; Ina-Salwany, Y. Prevalence, antimicrobial susceptibility and plasmid profiling of Vibrio spp. isolated from cultured groupers in Peninsular Malaysia. BMC Microbiol. 2019, 19, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverter, M.; Sarter, S.; Caruso, D.; Avarre, J.C.; Combe, M.; Pepey, E.; Pouyaud, L.; Vega-Heredia, S.; de Verdal, H.; Gozlan, R.E. Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat. Commun. 2020, 11, 1870. [Google Scholar] [CrossRef] [Green Version]
- CODEX Alimentarius. Guidelines on the Application of General Principles of Food Hygiene to the Control of pathogenic Vibrio in Seafood; CAC/GL 73-2010; FAO: Rome, Italy, 2010; 14p. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations); WHO (World Health Organization). Selection and Application of Methods for the Detection and Enumeration of Human-Pathogenic Halophilic Vibrio spp. in Seafood: Guidance; FAO and WHO: Geneva, Switzerland, 2016; 74p, Available online: https://apps.who.int/iris/handle/10665/249530 (accessed on 5 November 2021).
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e6406. [Google Scholar]
- CDC (Center for Disease Control and Prevention). Available online: https://www.cdc.gov/vibrio/faq.html (accessed on 5 November 2021).
- Slifka, K.M.J.; Newton, A.E.; Mahon, B.E. Vibrio alginolyticus infections in the USA, 1988–2012. Epidemiol. Infect. 2017, 145, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Korea Times. Available online: https://www.koreatimes.co.kr/www/nation/2020/09/119_295392.html (accessed on 5 November 2021).
- EFSA (EFASA Panel on Biological Hazards, BIOHAZ, EFSA Panel on Contaminants in the Food Chain, CONTAM). Scientific opinion on the minimum hygiene criteria to be applied to clean seawater and on the public health risks and hygiene criteria for bottled seawater intended for domestic use. EFSA J. 2012, 10, 2613. [Google Scholar] [CrossRef] [Green Version]
- Osunla, C.A.; Okoh, A.I. Vibrio pathogens: A public health concern in rural water resources in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2017, 14, 1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Kabore, S.; Cecchi, P.; Mosser, T.; Toubiana, M.; Traore, O.; Ouattara, A.S.; Traore, A.S.; Barro, N.; Colwell, R.R.; Monfort, P. Occurrence of Vibrio cholerae in water reservoirs of Burkina Faso. Res. Microbiol. 2018, 169, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Venohr, M.; Langhans, S.D.; Peters, O.; Holker, F.; Arlinghaus, R.; Mitchell, L.; Wolter, C. The underestimated dynamics and impacts of water-based recreational activities on freshwater ecosystems. Environ. Rev. 2018, 26, 199–213. [Google Scholar] [CrossRef] [Green Version]
- EFSA (EFSA Panel on Animal Health and Welfare). Scientific opinion on oyster mortality. EFSA J. 2015, 13, 4122. [Google Scholar] [CrossRef]
- Rigos, G.; Katharios, P.; Kogiannou, D.; Cascarano, C.M. Infectious diseases and treatment solutions of farmed greater amberjack Seriola dumerili with particular emphasis in Mediterranean region. Rev. Aquac. 2021, 13, 301–323. [Google Scholar] [CrossRef]
- Vezzulli, L.; Colwell, R.R.; Pruzzo, C. Ocean warming and spread of pathogenic Vibrios in the aquatic environment. Microb. Ecol. 2013, 65, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Hartnell, R.E.; Stockley, L.; Keay, W.; Rosec, J.P.; Hervio-Heath, D.; Van den Berg, H.; Leoni, F.; Ottaviani, D.; Henigman, U.; Denayer, S.; et al. A pan-European ring trial to validate an International Standard for detection of Vibrio cholerae, Vibrio parahaemolyticus and Vibrio vulnificus in seafoods. Int. J. Food Microbiol. 2019, 288, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Wiltshire, K.H.; Manly, B.F.J. The warming trend at Helgoland Roads, North Sea: Phytoplankton response. Helgol. Mar. Res. 2004, 58, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Hackbusch, S.; Wichels, A.; Gimenez, L.; Dopke, H.; Gerdts, G. Potentially human pathogenic Vibrio spp. in a coastal transect: Occurrence and multiple virulence factors. Sci. Total Environ. 2020, 707, 136113. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D. Vibrio vulnificus: New insights into a deadly opportunistic pathogen. Environ. Microbiol. 2018, 20, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Larsen, A.K.; Nymo, I.H.; Sorensen, K.K.; Seppola, M.; Rodven, R.; de Bagues, M.P.J.; Al Dahouk, S.; Godfroid, J. Concomitant temperature stress and immune activation may increase mortality despite efficient clearance of an intracellular bacterial infection in Atlantic Cod. Front. Microbiol. 2018, 9, 2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.F.; Chen, Y.W.; Xu, J.K.; Ding, W.Y.; Shao, A.Q.; Zhu, Y.T.; Wang, C.; Liang, X.; Yang, J.L. Temperature elevation and Vibrio cyclitrophicus infection reduce the diversity of haemolymph microbiome of the mussel Mytilus coruscus. Sci. Rep. 2019, 9, 16391. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.R.; Gast, R.J.; Fujioka, R.S.; Solo-Gabriele, H.M.; Meschke, J.S.; Amaral-Zettler, L.A.; del Castillo, E.; Polz, M.F.; Martin, F.; Collier, T.K.; et al. The coastal environment and human health: Microbial indicators, pathogens, sentinels and reservoirs. Environ. Health 2008, 7, S3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Vibrio Species | Environments | Hosts | Pathology |
|---|---|---|---|
| V. alginolyticus1 | seawater, human, seafood | human | wound, ear/eye and soft tissue infections |
| V. anguillarum | seawater, marine animals | salmon, bivalves | vibriosis, septicemic disease |
| V. carchariae | seawater, shark | shark?, human (shark bite) | human (shark bite) |
| V. cholerae1 | fresh and brackish waters, invertebrates, algae, aquatic animals | human | cholera: dehydration and electrolyte loss |
| V. corallilyticus2 | coral | zooxanthellae | coral bleaching |
| V. damsela3 | seawater, fish | fish, human | wound infections |
| V. fluvialis | water, oyster | human | diarrhea, gastroenteritis |
| V. furnissii | estuarine water | human | acute gastroenteritis (rare in clinic) |
| V. harveyi | seawater | shrimp, invertebrates, human | vibriosis |
| V. hollisae | water, seafood | human | diarrhea |
| V. mediterranei | seawater, coral endosymbiont | zooxanthellae | coral bleaching |
| V. metschnikovii | fresh, brackish, marine waters, seafood | human (rarely) | wound infections? |
| V. mimicus | water, seafood, birds | human | diarrhea |
| V. parahaemolyticuss1 | seafood | human | acute gastroenteritis |
| V. salmonicida4 | seawater, marine fishes | salmon, rainbow trout | cold-water vibriosis |
| V. shiloi | coral endosymbiont | zooxanthellae | coral bleaching |
| V. symbiont | pufferfish | toxic to humans and animals | |
| V. tubiashi | seawater, marine mollusks | Pacific oysters, bivalves | vibriosis |
| V. vulnificus1 | estuarine and seawaters, seafood, sediment | human | fulminant septicemia, wound infections |
| Niche | Density | Method | Vibrio spp. Prevalence (%) | Country [Ref.] |
|---|---|---|---|---|
| Port areas Ballast tanks Coastal waters | 1–4.4 × 103 UFC/mL 1–4.3 × 102 UFC/mL 2–1.6 × 103 UFC/mL | PCR-m | Vc (24); Vc O1 (ctxAC, tcpAC) Vc O1 (ctxAC, tcpAC) | Brazil [36] |
| FW BW | Not presented | MPN-PCR | Vc (34), Vm (19) Vp (51), Va (46), Vm (28) | South Africa [23] |
| TW Mud (M) | 1.4 × 101–5.0 × 104 MPN/mL 2.1 × 102–2.7 × 102 MPN/mL | Culture | Va, Vp Va (46), Vp, Vc, Vv | South Korea [37] |
| SW | 5 × 102–3.4 × 104 cells/mL | FISH | Vibrio spp. | Germany, North Sea [34] |
| EW S | 0–8.4 × 102 UFC/mL * 0–2.9 × 103 UFC/g * | Culture and PCR | Va and Vp (all year), Vv (summer), Vc (very low) | Germany, North Sea [35] |
| SW Lagoon | 0–log6.4 gene copies/100 mL * 0–log8.32 gene copies/100 mL * | Culture and real time PCR | Vv (14.9, July–September), Vc (33.6, June–September), Van (2.5, May and June), Vf (1.5, July) | Lithuania, Baltic Sea [38] |
| SW FW | Not presented | Culture | Vv (41), Vp (33), Va (15), Vn (4), and Vh (0.7) non-01 Vc (80), 01 Vc (13.1), Vm (2.6) | Iran, Caspian Sea [39] |
| SW | 0–1.2 × 105/L * | MPN-PCR | Vc, Vp, Vv | Guinea-Bissau [40] |
| EW | 2.3 × 105–2.4 × 1012 MPN/100 mL | MPN | 15 Vibrio spp.; Vp (16), Vc (14.5), Va (13) | Brazil [41] |
| Physiologic Characteristics | V. cholerae | V. parahaemolyticus | V. vulnificus |
|---|---|---|---|
| Temperature range (°C) | * 10–43 | 5–43 | 8–43 |
| Optimum temperature (°C) | 30–37 | 37 | 37 |
| Inactivation temperature (°C) | >70 | >63 | >50 |
| pH range | 5–9.7 | 5–11 | 5–10 |
| Optimum pH | 7.6 | 7.5–8.5 | 7.8 |
| Minimum water activity (aw) | 0.97 | 0.94 | 0.96 |
| NaCl (%) | <4.0 | 0.5–8.0 | 0.5–5.0 |
| Salinity (PSU) | 0–20 | 5–30 | 8–16 |
| Vibrio sp. | Gender | Age (Years) | Cause and Context | Symptoms | Country [Ref.] |
|---|---|---|---|---|---|
| V. alginolyticus | male male | 69 37 | Rectum adenocarcinoma receiving chemotherapy Pretibial ulcer exposed to the Mediterranean Sea | Watery diarrhea, abdominal pain, nausea, vomiting, no fever, lactic acidosis. Enterocolitis, septic shock Wound uncured | Spain [143] |
| V. cholera (non-O1 and non-O139) | male | 37 | Non-travel hepatitis C cirrhotic patient; shrimp consumption | Fever, abdominal pain, watery profuse diarrhea and dehydration | Portugal [144] |
| V. cholera (strain not mentioned) | female | 70 | Ovarian carcinoma (stage IIIC) receiving chemotherapy; shrimp consumption | Nausea, vomiting, abdominal pain. Pancytopenia, lactic acidosis, hypotension, oral/nasal hemorrhage, coagulopathy. Death | USA [145] |
| V. cholera (O100, virulence genes hapA, hlyA, and ompU) | male | 27 | A water skiing accident on a river in Australia | Chronic periodic earaches along purulent exudate, otitis | Israel [146] |
| V. cholera (non-O1) | male | 59 | Fisherman struck in the right eye by a marine shrimp developed keratitis | Right eye pain, redness, tearing and photophobia, corneal ulcer | Taiwan [147] |
| V. cholera (non-O1) | male | 27 | Immunocompetent patient; denied exposure to marine water, marine animals and eating raw seafood; tap water and well water contaminated | Abdominal pain, nausea, vomiting, chills and dysuria. Afebrile, stable vital signs; mild lower abdominal tenderness. Urinary tract infection | Lebanon [142] |
| V. harvey | male | 26 | Wound infection following a traumatic leg amputation during snorkeling | Fever, putrid infection and necrosis of the subcutaneous tissue and the muscles | Spain [148] |
| V. harvey and P. damselae | male | 75 | Fishing at a southern Sydney beach. Not recall any hand trauma. Treated for hypertension and hypercholesterolemia | Enlarging, painful hemorrhagic blister on his right hand | Australia [149] |
| V. metschnikovii | male | 78 | Previous gastroenteritis after shrimp consumption | Pain, swelling and redness of his left dorsalis pedis, fever mental confusion, severe septic shock and cardiac arrest. Need of mechanical ventilation | Denmark [150] |
| V. metschnikovii | male | 70 | Heart failure, atherosclerosis, vascular prosthesis, chronic gastric ulcer and kidney disease, gastrointestinal bleeding | Intestinal bleeding, pseudoaneurysm of aorto-bifemoral graft and aorto- small intestine fistula formation; hospital-acquired bilateral pneumonia; post-chirurgical infection | Ukraine [151] |
| V. parahaemolyticus | male | 85 | Severe valvular and ischemic-hypertensive cardiopathy, slight chronic renal failure and chronic venous leg ulcers; sea beach holiday in Italy. Chronic hepatitis B (undiagnosed) | Pain, limb cellulitis originating from leg ulcers, hypotension, acute renal failure, septicemia and septic shock | Switzerland [152] |
| V. vulnificus | male | 39 | Immunosuppressive therapy, admitted to the hospital for liver transplantation | Fever, myalgia, anuria and erythematous plaques on lower limbs, dyspnea and respiratory distress. Death | Brazil [153] |
| V. vulnificus | male | 46 | History of a remote Roux-en-Y gastric bypass; ingestion of raw oysters | Distress, leg soft tissue necrosis, multi-organ failure, mechanical ventilation, disseminated intra-vascular coagulopathy, large ischemic stroke. Death | USA [154] |
| V. vulnificus | male | 31 | Chronic liver disease—cirrhosis; leg tattoo exposure to seawater, Gulf of Mexico | Fever, erythema in tattoo area and in legs, cellulitis, septic shock. Death | USA [155] |
| P. damselae * | male | 65 | Trauma in right arm during fish cleaning; arterial venous fistula due blood dialysis | Pain and edema in the arm, necrotizing fasciitis | Portugal [156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampaio, A.; Silva, V.; Poeta, P.; Aonofriesei, F. Vibrio spp.: Life Strategies, Ecology, and Risks in a Changing Environment. Diversity 2022, 14, 97. https://doi.org/10.3390/d14020097
Sampaio A, Silva V, Poeta P, Aonofriesei F. Vibrio spp.: Life Strategies, Ecology, and Risks in a Changing Environment. Diversity. 2022; 14(2):97. https://doi.org/10.3390/d14020097
Chicago/Turabian StyleSampaio, Ana, Vanessa Silva, Patrícia Poeta, and Florin Aonofriesei. 2022. "Vibrio spp.: Life Strategies, Ecology, and Risks in a Changing Environment" Diversity 14, no. 2: 97. https://doi.org/10.3390/d14020097
APA StyleSampaio, A., Silva, V., Poeta, P., & Aonofriesei, F. (2022). Vibrio spp.: Life Strategies, Ecology, and Risks in a Changing Environment. Diversity, 14(2), 97. https://doi.org/10.3390/d14020097