Cryptic Triploids and Leaky Premating Isolation in an Odontophrynus Hybrid Zone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Localities and Sampling Design
2.2. Recording and Analysis of Advertisement Calls
2.3. Erythrometric Ploidy Determination and Morphometry
2.4. Statistical Analyses
3. Results
3.1. Odontophrynus Species Composition at the Study Localities
3.2. Morphometric Features of Odontophrynus Taxa at the La Escondida Study Site
3.3. Advertisement Call Features of Odontophrynus Taxa
3.4. Advertisement Call Features of Allopatric and Sympatric Odontophrynus Populations
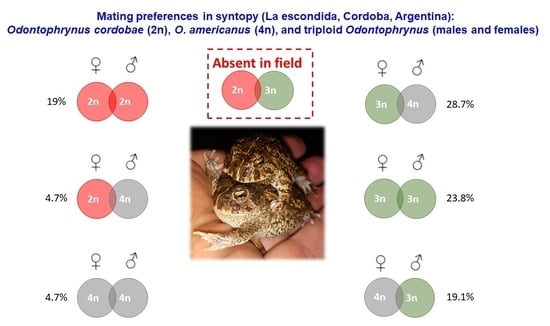
3.5. Mating Preferences at the La Escondida Contact Zone
4. Discussion
4.1. Prediction 1: Hybridization Due to Natural Mating between the Diploid O. cordobae and Tetraploid O. americanus Is Rare to Absent
4.2. Prediction 2: Advertisement Call Structure of Triploids Resembles More O. americanus than O. cordobae
4.3. Prediction 3: Frequent Mismatings Occur between O. americanus and Triploid Specimens, but Not with O. cordobae
4.4. Putative Origin of Triploids and Their Local Long-Term Persistence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, B.J.; Pyron, R.A.; Wiens, J.J. Polyploidization and Sex Chromosome Evolution in Amphibians. In Polyploidy and Genome Evolution; Soltis, P.S., Soltis, D.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 385–410. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference; Electronic Database; Version 6.1; American Museum of Natural History: New York, NY, USA, 2021; Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 28 February 2021).
- Evans, B.J.; Carter, T.F.; Greenbaum, E.; Gvoždík, V.; Kelley, D.B.; McLaughlin, P.J.; Pauwels, O.S.G.; Portik, D.M.; Stanley, E.L.; Tinsley, R.C.; et al. Genetics, Morphology, Advertisement Calls, and Historical Records Distinguish Six New Polyploid Species of African Clawed Frog (Xenopus, Pipidae) from West and Central Africa. PLoS ONE 2015, 10, e0142823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beçak, M.L.; Beçak, W.; Rabello, M.N. Cytological evidence of constant tetraploidy in the bisexual South American frog Odontophrynus americanus. Chromosoma 1966, 19, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Stöck, M.; Frynta, D.; Grosse, W.-R.; Steinlein, C.; Schmid, M. A review of the distribution of diploid, triploid and tetraploid green toads (Bufo viridis complex) in Asia including new data from Iran and Pakistan. Asiat. Herpetol. Res. 2001, 9, 77–100. [Google Scholar] [CrossRef]
- Stöck, M.; Moritz, C.; Hickerson, M.; Frynta, D.; Dujsebayeva, T.; Eremchenko, V.; Macey, J.R.; Papenfuss, T.J.; Wake, D.B. Evolution of mitochondrial relationships and biogeography of Palearctic green toads (Bufo viridis subgroup) with insights in their genomic plasticity. Mol. Phylogenetics Evol. 2006, 41, 663–689. [Google Scholar] [CrossRef] [Green Version]
- Stöck, M.; Roth, P.; Podloucky, R.; Grossenbacher, K. Wechselkröten unter Berücksichtigung von Bufo viridis viridis Laurenti, 1768; Bufo variabilis (Pallas, 1769); Bufo boulengeri Lataste, 1879; Bufo balearicus Böttger, 1880 und Bufo siculus Stöck, Sicilia, Belfiore, Lo Brutto, Lo Valvo und Arculeo, 2008. In Handbuch der Amphibien und Reptilien Europas; Grossenbacher, K., Ed.; Aula Verlag: Wiebelsheim, Germany, 2009; Volume 5, pp. 413–498. [Google Scholar]
- Stöck, M.; Ustinova, J.; Lamatsch, D.K.; Schartl, M.; Perrin, N.; Moritz, C. A vertebrate reproductive system involving three ploidy levels: Hybrid origin of triploids in a contact zone of diploid and tetraploid palearctic green toads (Bufo viridis subgroup). Evolution 2010, 64, 944–959. [Google Scholar] [CrossRef]
- Stöck, M.; Ustinova, J.; Betto-Colliard, C.; Schartl, M.; Moritz, C.; Perrin, N. Simultaneous Mendelian and clonal genome transmission in a sexually reproducing, all-triploid vertebrate. Proc. R. Soc. B-Biol. Sci. 2012, 279, 1293–1299. [Google Scholar] [CrossRef] [Green Version]
- Mable, B.K.; Alexandrou, M.A.; Taylor, M.I. Genome duplication in amphibians and fish: An extended synthesis. J. Zool. 2011, 284, 151–182. [Google Scholar] [CrossRef]
- Choleva, L.; Janko, K. Rise and Persistence of Animal Polyploidy: Evolutionary Constraints and Potential. Cytogenet. Genome Res. 2013, 140, 151–170. [Google Scholar] [CrossRef]
- Dufresnes, C.; Mazepa, G.; Jablonski, D.; Oliveira, R.C.; Wenseleers, T.; Shabanov, D.A.; Auer, M.; Ernst, R.; Koch, C.; Ramirez-Chaves, H.E.; et al. Fifteen shades of green: The evolution of Bufotes toads revisited. Mol. Phylogenetics Evol. 2019, 141, 106615. [Google Scholar] [CrossRef]
- Castellano, S.; Giacoma, C.; Dujsebayeva, T.; Odierna, G.; Balletto, E. Morphometrical and acoustical comparison between diploid and tetraploid green toads. Biol. J. Linn. Soc. 1998, 63, 257–281. [Google Scholar] [CrossRef]
- Gerhardt, H.C.; Tucker, M.A.; von Twickel, A.; Walkowiak, W. Anuran Vocal Communication: Effects of Genome Size, Cell Number and Cell Size. Brain Behav. Evol. 2022, 96, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H. Mating calls of autotriploid and autotetraploid males in Hyla japonica. Sci. Rep. Lab. Amphib. Biol. Hiroshima Univ. 1993, 12, 177–189. [Google Scholar]
- Gerhardt, H.C. Advertisement-call preferences in diploid-tetraploid treefrogs (Hyla chrysoscelis and Hyla versicolor): Implications for mate choice and the evolution of communication systems. Evolution 2005, 59, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Guignard, M.; Buechi, L.; Getaz, M.; Betto-Colliard, C.; Stöck, M. Genome size rather than content might affect call properties in toads of three ploidy levels (Anura: Bufonidae: Bufo viridis subgroup). Biol. J. Linn. Soc. 2012, 105, 584–590. [Google Scholar] [CrossRef] [Green Version]
- Martino, A.L.; Sinsch, U. Speciation by polyploidy in Odontophrynus americanus. J. Zool. 2002, 257, 67–81. [Google Scholar] [CrossRef]
- Grenat, P. Identificación Geográfica de Zonas Híbridas y Determinación de Diferencias Interespecíficas de Poblaciones Diplo/Tetraploides de Odontophrynus cordobae y Odontophrynus americanus. Ph.D. Thesis, Universidad Nacional de Rio Cuarto, Rio Cuarto, Argentina, 2006. [Google Scholar]
- Grenat, P.; Salas, N.; Pollo, F.; Otero, M.; Baraquet, M.; Sinsch, U.; Martino, A. Naturally occurring triploids in contact zones between diploid/tetraploid closely related species Odontophrynus cordobae and O. americanus (Anura, Cycloramphidae). Amphibia-Reptilia 2018, 39, 1–10. [Google Scholar] [CrossRef]
- Beçak, W.; Beçak, M.L.; de Langlada, F.G. Artificial triploid hybrids by interspecific mating of Odontophrynus (Amphibia, Anura). Experientia 1968, 24, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Beçak, M.L.; Becak, W. Further studies on polyploid amphibians (Ceratophrydidae). III. Meiotic aspects of the Interspecific triploid hybrid. Chromosoma 1970, 31, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, I.R.G.; Bonaldo, M.F.; Beçak, W. In situ localization of ribosomal genes in a natural triploid of Odontophrynus. J. Hered. 1980, 71, 55–57. [Google Scholar] [CrossRef]
- Rosset, S.D.; Baldo, D.; Lanzone, C.; Basso, N.G. Review of the geographic distribution of diploid and tetraploid populations of the Odontophrynus americanus species complex (Anura: Leptodactylidae). J. Herpetol. 2006, 40, 465–477. [Google Scholar] [CrossRef]
- Otero, M.A.; Grenat, P.R.; Bionda, C.L.; Baraquet, M.; Pollo, F.E.; Salas, N.E.; Martino, A.L. Age and growth in an anuran hybrid zone: Fitness-related traits of the diploid/polyploid ground frog complex (genus Odontophrynus) from central Argentina. Zool. Anz. 2021, 293, 257–262. [Google Scholar] [CrossRef]
- Grenat, P.R.; Valetti, J.A.; Martino, A.L. Call variability, stereotypy and relationships in syntopy of tetraploid common lesser escuerzo (Anura: Genus Odontophrynus). Zool. Anz.-J. Comp. Zool. 2017, 268, 143–150. [Google Scholar] [CrossRef]
- Grenat, P.R.; Valetti, J.A.; Martino, A.L. Intra-specific variation in advertisement call of Odontophrynus cordobae (Anura, Cycloramphidae): A multilevel and multifactor analysis. Amphibia-Reptilia 2013, 34, 471–482. [Google Scholar] [CrossRef]
- Nöller, H.G. Eine einfache Technik der Blutentnahme beim Frosch. Pflüg. Arch. Gesamte Physiol. Menschen Tiere 1959, 269, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Grenat, P.; Salas, N.E.; Martino, A.L. Erythrocyte size as diagnostic character for the identification of live cryptic Odontophrynus americanus and O. cordobae (Anura: Cycloramphidae). Zootaxa 2009, 2049, 67–68. [Google Scholar] [CrossRef]
- Otero, M.A.; Grenat, P.R.; Valetti, J.A.; Salas, N.E.; Martino, A.L. Erythrocyte nuclear size as a better diagnostic character than cell size in the identification of live cryptic polyploid species. Zootaxa 2013, 3694, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Salas, N. Análisis cromosómico de Odontophrynus americanus, O. achalensis, O. cordobae y O. occidentalis (Anura, Leptodactylidae) de la provincia de Córdoba, Argentina. Rev. Esp. Herpetol. 2006, 20, 31–38. [Google Scholar]
- Heyer, W.R.; Rand, A.S.; Da Cruz, C.A.G.; Peixoto, O.L.; Nelson, C.E. Frogs of Boracéia. Arq. Zool. 1990, 31, 231–410. [Google Scholar]
- Grenat, P.R.; Salas, N.E.; Martino, A.L. Variación morfométrica intra e interespecífica entre poblaciones de Odontophrynus (Anura: Cycloramphidae) del área central de Argentina. Rev. Biol. Trop. 2012, 60, 1589–1601. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, H.C.; Ptacek, M.B.; Barnett, L.; Torke, K.G. Hybridization in the Diploid-Tetraploid Treefrogs Hyla chrysoscelis and Hyla versicolor. Copeia 1994, 1994, 51–59. [Google Scholar] [CrossRef]
- Stöck, M.; Schmid, M.; Steinlein, C.; Grosse, W.R. Mosaicism in somatic triploid specimens of the Bufo viridis complex in the Karakoram with examination of calls, morphology and taxonomic conclusions. Ital. J. Zool. 1999, 66, 215–232. [Google Scholar] [CrossRef] [Green Version]
- Mable, B.K.; Bogart, J.P. Call analysis of triploid hybrids resulting from diploid-tetraploid species crosses of hylid tree frogs. Bioacoustics 1991, 3, 111–119. [Google Scholar] [CrossRef]
- Stöck, M.; Lamatsch, D.K.; Steinlein, C.; Epplen, J.T.; Grosse, W.R.; Hock, R.; Klapperstück, T.; Lampert, K.P.; Scheer, U.; Schmid, M.; et al. A bisexually reproducing all-triploid vertebrate. Nat. Genet. 2002, 30, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Slaby, S.; Titran, P.; Marchand, G.; Hanotel, J.; Lescuyer, A.; Lepretre, A.; Bodart, J.F.; Marin, M.; Lemiere, S. Effects of glyphosate and a commercial formulation Roundup (R) exposures on maturation of Xenopus laevis oocytes. Environ. Sci. Pollut. Res. 2020, 27, 3697–3705. [Google Scholar] [CrossRef] [PubMed]





| Locality | Latitude Longitude | Altitude (m a.s.l.) | Distance (km) | Odontophrynus Taxa |
|---|---|---|---|---|
| Villa General Belgrano | 31°58′ S 64°32′ W | 730 | 76 | O. cordobae (2n) |
| Rio de los Sauces | 32°32′ S 64°36′ W | 734 | 15 | O. cordobae (2n) O. americanus (4n) |
| El Cano | 32°37′ S 64°35′ W | 724 | 6 | O. cordobae (2n) O. americanus (4n) |
| Escuela La Escondida | 32°40′ S 64°32′ W | 690–740 | 0 | O. cordobae (2n) O. americanus (4n) Odontophrynus (3n) |
| Coronel Baigorria | 32°51′ S 64°20′ W | 509 | 32 | O. americanus (4n) |
| Rio Cuarto | 33°06′ S 64°20′ W | 458 | 57 | O. americanus (4n) |
| Locality | O. cordobae (2n) | O. americanus (4n) | Odontophrynus (3n) | |||
|---|---|---|---|---|---|---|
| Erythrocytes | Advertisement Calls | Erythrocytes | Advertisement Calls | Erythrocytes | Advertisement Calls | |
| Villa General Belgrano | 17 | 38 | - | - | - | - |
| Rio de los Sauces | 12 | 15 | - | 2 | - | - |
| El Cano | 6 | 7 | 13 | - | - | - |
| Escuela La Escondida | 53 | 28 | 57 | 51 | 77 | 38 |
| Coronel Baigorria | - | - | 14 | 13 | - | - |
| Rio Cuarto | - | - | 28 | 45 | - | - |
| O. cordobae ♂ | O. americanus ♂ | O. 3n ♂ | |
|---|---|---|---|
| O. cordobae ♀ | 4 (19.0%) | 1 (4.7%) | - |
| O. americanus ♀ | - | 1 (4.7%) | 4 (19.0%) |
| O. 3n ♀ | - | 6 (28.7%) | 5 (23.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martino, A.L.; Grenat, P.R.; Sinsch, U. Cryptic Triploids and Leaky Premating Isolation in an Odontophrynus Hybrid Zone. Diversity 2022, 14, 305. https://doi.org/10.3390/d14040305
Martino AL, Grenat PR, Sinsch U. Cryptic Triploids and Leaky Premating Isolation in an Odontophrynus Hybrid Zone. Diversity. 2022; 14(4):305. https://doi.org/10.3390/d14040305
Chicago/Turabian StyleMartino, Adolfo L., Pablo R. Grenat, and Ulrich Sinsch. 2022. "Cryptic Triploids and Leaky Premating Isolation in an Odontophrynus Hybrid Zone" Diversity 14, no. 4: 305. https://doi.org/10.3390/d14040305
APA StyleMartino, A. L., Grenat, P. R., & Sinsch, U. (2022). Cryptic Triploids and Leaky Premating Isolation in an Odontophrynus Hybrid Zone. Diversity, 14(4), 305. https://doi.org/10.3390/d14040305