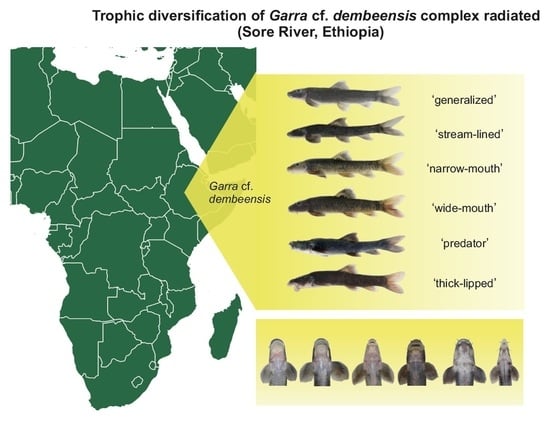
Trophic Diversification Out of Ancestral Specialization: An Example from a Radiating African Cyprinid Fish (Genus Garra)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Diet and Stable Isotopes
2.3. Statistical Analyses
3. Results
3.1. Gut Length
3.2. Diet
3.3. Stable Isotopes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schluter, D. The Ecology of Adaptive Radiation; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Meyer, A. Phylogenetic relationships and evolutionary processes in East African cichlid fishes. Trends Ecol. Evol. 1993, 8, 279–284. [Google Scholar] [CrossRef]
- Nagelkerke, L.A.J.; Sibbing, F.A.; van den Boogaart, J.G.M.; Lammens, E.H.R.R.; Osse, J.W.M. The barbs (Barbus spp.) of Lake Tana: A forgotten species flock? Environ. Biol. Fishes 1994, 39, 1–22. [Google Scholar] [CrossRef]
- Mina, M.V.; Mironovsky, A.N.; Dgebuadze, Y.U. Lake Tana large barbs: Phenetics, growth and diversification. J. Fish Biol. 1996, 48, 383–404. [Google Scholar] [CrossRef]
- Seehausen, O.; Wagner, C.E. Speciation in freshwater fishes. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 621–651. [Google Scholar] [CrossRef]
- Alekseyev, S.S.; Bajno, R.; Gordeeva, N.V.; Reist, J.D.; Power, M.; Kirillov, A.F.; Samusenok, V.P.; Matveev, A.N. Phylogeography and sympatric differentiation of the Arctic charr Salvelinus alpinus (L.) complex in Siberia as revealed by mtDNA sequence analysis. J. Fish Biol. 2009, 75, 368–392. [Google Scholar] [CrossRef]
- Østbye, K.; Amundsen, P.-A.; Bernatchez, L.; Klemetsen, A.; Knudsen, R.; Kristoffersen, R.; Naesje, T.F.; Hindar, K. Parallel evolution of ecomorphological traits in the European whitefish Coregonus lavaretus (L.) species complex during postglacial times. Mol. Ecol. 2006, 15, 3983–4001. [Google Scholar] [CrossRef]
- Præbel, K.; Knudsen, R.; Siwertsson, A.; Karhunen, M.; Kahilainen, K.K.; Ovaskainen, O.; Østbye, K.; Peruzzi, S.; Fevolden, S.; Amundsen, P. Ecological speciation in postglacial European whitefish: Rapid adaptive radiations into the littoral, pelagic, and profundal lake habitats. Ecol. Evol. 2013, 3, 4970–4986. [Google Scholar] [CrossRef]
- Jacobs, A.; Carruthers, M.; Yurchenko, A.; Gordeeva, N.V.; Alekseyev, S.S.; Hooker, O.; Leong, J.S.; Minkley, D.R.; Rondeau, E.B.; Koop, B.F.; et al. Parallelism in eco-morphology and gene expression despite variable evolutionary and genomic backgrounds in a Holarctic fish. PLoS Genet. 2020, 16, e1008658. [Google Scholar] [CrossRef]
- Levin, B.; Simonov, E.; Gabrielyan, B.K.; Mayden, R.L.; Rastorguev, S.M.; Roubenyan, H.R.; Sharko, F.S.; Nedoluzhko, A.V. Caucasian treasure: Genomics sheds light on the evolution of half-extinct Sevan trout, Salmo ischchan, species flock. Mol. Phylogenetics Evol. 2022, 167, 107346. [Google Scholar] [CrossRef]
- Masonick, P.; Meyer, A.; Hulsey, C.D. Phylogenomic Analyses Show Repeated Evolution of Hypertrophied Lips Among Lake Malawi Cichlid Fishes. Genome Biol. Evol. 2022, 14, evac051. [Google Scholar] [CrossRef]
- Barluenga, M.; Stölting, K.N.; Salzburger, W.; Muschick, M.; Meyer, A. Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 2006, 439, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Elmer, K.R.; Fan, S.; Kusche, H.; Luise Spreitzer, M.; Kautt, A.F.; Franchini, P.; Meyer, A. Parallel evolution of Nicaraguan crater lake cichlid fishes via non-parallel routes. Nat. Commun. 2014, 5, 5168. [Google Scholar] [CrossRef] [PubMed]
- Burress, E.D.; Piálek, L.; Casciotta, J.R.; Almirón, A.; Tan, M.; Armbruster, J.W.; Říčan, O. Island-and lake-like parallel adaptive radiations replicated in rivers. Proc. R. Soc. B Biol. Sci. 2018, 285, 20171762. [Google Scholar] [CrossRef] [PubMed]
- Muschick, M.; Nosil, P.; Roesti, M.; Dittmann, M.T.; Harmon, L.; Salzburger, W. Testing the stages model in the adaptive radiation of cichlid fishes in East African Lake Tanganyika. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140605. [Google Scholar] [CrossRef] [PubMed]
- Rüber, L.; Verheyen, E.; Meyer, A. Replicated evolution of trophic specializations in an endemic cichlid fish lineage from Lake Tanganyika. Proc. Nat. Acad. Sci. USA 1999, 96, 10230–10235. [Google Scholar] [CrossRef]
- Alekseyev, S.S.; Samusenok, V.P.; Matveev, A.N.; Pichugin, M.Y. Diversification, Sympatric Speciation, and Trophic Polymorphism of Arctic Charr, Salvelinus Alpinus Complex, in Transbaikalia. Env. Biol. Fish 2002, 64, 97–114. [Google Scholar] [CrossRef]
- Terekhanova, N.V.; Logacheva, M.D.; Penin, A.A.; Neretina, T.V.; Barmintseva, A.E.; Bazykin, G.A.; Kondrashov, A.S.; Mugue, N.S. Fast evolution from precast bricks: Genomics of young freshwater populations of threespine stickleback Gasterosteus aculeatus. PLoS Genet. 2014, 10, e1004696. [Google Scholar] [CrossRef]
- Brodersen, J.; Post, D.M.; Seehausen, O. Upward adaptive radiation cascades: Predator diversification induced by prey diversification. Trends Ecol. Evol. 2018, 33, 59–70. [Google Scholar] [CrossRef]
- Esin, E.V.; Bocharova, E.S.; Borisova, E.A.; Markevich, G.N. Interaction among morphological, trophic and genetic groups in the rapidly radiating Salvelinus fishes from Lake Kronotskoe. Evol. Ecol. 2020, 34, 611–632. [Google Scholar] [CrossRef]
- Skúlason, S. Sympatric morphs, populations and speciation in freshwater fish with emphasis on arctic charr. In Evolution of Biological Diversity; Magurran, A., May, R.M., Eds.; Oxford University Press: New York, NY, USA, 1999; pp. 71–92. [Google Scholar]
- Mina, M.V.; Mironovsky, A.N.; Golubtsov, A.S.; Dgebuadze, Y.Y., II. Morphological diversity of “large barbs”; from Lake Tana and neighbouring areas: Homoplasies or synapomorphies? Ital. J. Zool. 1998, 65, 9–14. [Google Scholar] [CrossRef]
- Dgebuadze, Y.Y.; Mina, M.V.; Alekseyev, S.S.; Golubtsov, A.S. Observations on reproduction of the Lake Tana barbs. J. Fish Biol. 1999, 54, 417–423. [Google Scholar] [CrossRef]
- Dgebuadze, Y.Y.; Mironovsky, A.N.; Mendsaikhan, B.; Slyn’ko, Y.V. Rapid Morphological Diversification of the Cyprinid Fish Oreoleuciscus potanini (Cyprinidae) in the Course of Formation of a Reservoir in a River of the Semiarid Zone. Dokl. Biol. Sci. 2020, 490, 12–15. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, M.; Dejen, E.; Osse, J.W.M.; Sibbing, F.A. Adaptive radiation of Lake Tana’s (Ethiopia) Labeobarbus species flock (Pisces, Cyprinidae). Mar. Freshw. Res. 2008, 59, 391–407. [Google Scholar] [CrossRef]
- Komarova, A.S.; Rozanova, O.L.; Levin, B.A. Trophic resource partitioning by sympatric ecomorphs of Schizopygopsis (Cyprinidae) in a young Pamir Mountain lake: Preliminary results. Ichthyol. Res. 2021, 68, 191–197. [Google Scholar] [CrossRef]
- Levin, B.A.; Casal-López, M.; Simonov, E.; Dgebuadze, Y.Y.; Mugue, N.S.; Tiunov, A.V.; Doadrio, I.; Golubtsov, A.S. Adaptive radiation of barbs of the genus Labeobarbus (Cyprinidae) in an East African river. Freshw. Biol. 2019, 64, 1721–1736. [Google Scholar] [CrossRef]
- Levin, B.A.; Komarova, A.S.; Rozanova, O.L.; Golubtsov, A.S. Unexpected Diversity of Feeding Modes among Chisel-Mouthed Ethiopian Labeobarbus (Cyprinidae). Water 2021, 13, 2345. [Google Scholar] [CrossRef]
- Sibbing, F.A.; Nagelkerke, L.A.; Stet, R.J.; Osse, J.W. Speciation of endemic Lake Tana barbs (Cyprinidae, Ethiopia) driven by trophic resource partitioning; a molecular and ecomorphological approach. Aquat. Ecol. 1998, 32, 217–227. [Google Scholar] [CrossRef]
- Shkil, F.N.; Levin, B.A.; Abdissa, B.; Smirnov, S.V. Variability in the number of tooth rows in the pharyngeal dentition of Barbus intermedius (Teleostei; Cyprinidae): Genetic, hormonal and environmental factors. J. Appl. Ichthyol. 2010, 26, 315–319. [Google Scholar] [CrossRef]
- Shkil, F.N.; Lazebnyi, O.E.; Kapitanova, D.V.; Abdissa, B.; Borisov, V.B.; Smirnov, S.V. Ontogenetic mechanisms of explosive morphological divergence in the Lake Tana (Ethiopia) species flock of large African barbs (Labeobarbus; Cyprinidae; Teleostei). Russ. J. Dev. Biol. 2015, 46, 294–306. [Google Scholar] [CrossRef]
- Dimmick, W.W.; Berendzen, P.B.; Golubtsov, A.S. Genetic comparison of three Barbus (Cyprinidae) morphotypes from the Genale River, Ethiopia. Copeia 2001, 4, 1123–1129. [Google Scholar] [CrossRef]
- Levin, B.A.; Simonov, E.; Dgebuadze, Y.Y.; Levina, M.; Golubtsov, A.S. In the rivers: Multiple adaptive radiations of cyprinid fishes (Labeobarbus) in Ethiopian Highlands. Sci. Rep. 2020, 10, 7192. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.; Simonov, E.; Franchini, P.; Mugue, N.; Golubtsov, A.; Meyer, A. Rapid adaptive radiation in a hillstream cyprinid fish in the East African White Nile River basin. Mol. Ecol. 2021, 30, 5530–5550. [Google Scholar] [CrossRef] [PubMed]
- Melnik, N.O.; Esin, E.V. Skull morphology variation as related to trophic specialization in three forms of Salvelinus malma (Salmonidae) from the Kamchatka River basin. Dokl. Biol. Sci. 2020, 492, 75–78. [Google Scholar] [CrossRef]
- Piálek, L.; Říčan, O.; Casciotta, J.; Almirón, A.; Zrzavý, J. Multilocus phylogeny of Crenicichla (Teleostei: Cichlidae), with biogeography of the C. lacustris group: Species flocks as a model for sympatric speciation in rivers. Mol. Phylogenetics Evol. 2012, 62, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.R.; Khaironizam, M.Z. Trophic polymorphism in the Malaysian fish Neolissochilus soroides and other Old world barbs (Teleostei, Cyprinidae). Nat. Hist. Bull. Siam Soc. 2008, 56, 25–53. [Google Scholar]
- Schwarzer, J.; Misof, B.; Ifuta, S.N.; Schliewen, U.K. Time and origin of cichlid colonization of the lower Congo rapids. PLoS ONE 2011, 6, e22380. [Google Scholar] [CrossRef]
- Golubtsov, A.S.; Cherenkov, S.E.; Tefera, F.T. High morphological diversity of the genus Garra in the Sore River (the White Nile Basin, Ethiopia): One more cyprinid species flock? J. Ichthyol. 2012, 52, 817–820. [Google Scholar] [CrossRef]
- Kottelat, M. Ceratogarra, a genus name for Garra cambodgiensis and G. fasciacauda and comments on the oral and gular soft anatomy in labeonine fishes (Teleostei: Cyprinidae). Raffles Bull. Zool. Suppl. 2020, 35, 156–178. [Google Scholar] [CrossRef]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. (Eds.) Eschmeyer’s Catalog of Fishes: Genera, Species, References. 2022. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 15 February 2022).
- Menon, A.G.K. Monograph of the cyprinid fishes of the genus Garra, Hamilton. Mem. Indian. Mus. 1964, 14, 173–260. [Google Scholar]
- Zhang, E. Garra bispinosa, a new species of cyprinid fish (Teleostei: Cypriniformes) from Yunnan, Southwest China. Raffles Bull. Zool. 2005, 13, 9–15. [Google Scholar]
- Hamidan, N.; Jackson, M.C.; Britton, J.R. Diet and trophic niche of the endangered fish Garra ghorensis in three Jordanian populations. Ecol. Freshw. Fish 2016, 25, 455–464. [Google Scholar] [CrossRef]
- Dibaba, A.; Soromessa, T.; Workineh, B. Carbon stock of the various carbon pools in Gerba-Dima moist Afromontane forest, South-western Ethiopia. Carbon Balance Manag. 2019, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Kebede, A.; Diekkrüger, B.; Moges, S.A. Comparative study of a physically based distributed hydrological model versus a conceptual hydrological model for assessment of climate change response in the Upper Nile, Baro-Akobo basin: A case study of the Sore watershed, Ethiopia. Int. J. River Basin Manag. 2014, 12, 299–318. [Google Scholar] [CrossRef]
- Topic Popovic, N.; Strunjak-Perovic, I.; Coz-Rakovac, R.; Barisic, J.; Jadan, M.; Persin Berakovic, A.; Sauerborn Klobucar, R. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J. Appl. Ichthyol. 2012, 28, 553–564. [Google Scholar] [CrossRef]
- Google Maps. 2022. Available online: https://www.google.com/maps (accessed on 30 June 2022).
- Wagner, C.E.; McIntyre, P.B.; Buels, K.S.; Gilbert, D.M.; Michel, E. Diet predicts intestine length in Lake Tanganyika’s cichlid fishes. Funct. Ecol. 2009, 23, 1122–1131. [Google Scholar] [CrossRef]
- Ewart-Smith, J.L. The Relationship between Periphyton, Flow and Nutrients in Foothill Rivers of the South-Western Cape, South Africa. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2012. [Google Scholar]
- Natarajan, A.V.; Jhingran, A.G. Index of preponderance—A method of grading the food elements in the stomach analysis of fishes. Indian J. Fish 1961, 8, 54–59. [Google Scholar]
- Popova, O.A.; Reshetnikov, Y.S. On Complex Indices in Investigation of Fish Feeding. J. Ichthyol. 2011, 51, 686–691. [Google Scholar] [CrossRef]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montana, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2021; Available online: http://www.rstudio.com (accessed on 12 February 2021).
- Comtois, D. Summarytools: Tools to Quickly and Neatly Summarize Data. R Package Version 0.8. 72018. 2018. Available online: https://CRAN.R-project.org/package=summarytools (accessed on 12 February 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Pohlert, T. Package ‘PMCMRplus’. R Package Version 1.9.2.2021. Available online: https://cran.r-project.org/web/packages/PMCMRplus/index.html (accessed on 15 April 2022).
- Jackson, A.L.; Parnell, A.C.; Inger, R.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER0–Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Swanson, H.K.; Lysy, M.; Power, M.; Stasko, A.D.; Johnson, J.D.; Reist, J.D. A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 2015, 96, 318–324. [Google Scholar] [CrossRef]
- Matthes, H.A. Comparative study of the feeding mechanisms of some African Cyprinidae (Pisces, Cypriniformes). Bijdr. Dierk. 1963, 33, 3–35. [Google Scholar] [CrossRef]
- Levin, B.A.; Freyhof, J.; Lajbner, Z.; Perea, S.; Abdoli, A.; Gaffaroğlu, M.; Özuluğ, M.; Rubenyan, H.R.; Salnikov, V.B.; Doadrio, I. Phylogenetic relationships of the algae scraping cyprinid genus Capoeta (Teleostei: Cyprinidae). Mol. Phylogenetics Evol. 2012, 62, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Hata, H.; Shibata, J.; Omori, K.; Kohda, M.; Hori, M. Depth segregation and diet disparity revealed by stable isotope analyses in sympatric herbivorous cichlids in Lake Tanganyika. Zool. Lett. 2015, 1, 1–11. [Google Scholar] [CrossRef]
- Azim, M.E.; Beveridge, M.C.M.; van Dam, A.A.; Verdegem, M.C. Periphyton and aquatic production: An introduction. In Periphyton: Ecology, Exploitation and Management; Azim, M.E., Verdegem, M.C.J., van Dam, A.A., Beveridge, M.C.M., Eds.; Centre for Agriculture and Bioscience International: Wallingford, UK, 2005; pp. 1–13. [Google Scholar]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Saikia, S.K. Review on periphyton as mediator of nutrient transfer in aquatic ecosystems. Ecol. Balk. 2011, 3, 65–78. [Google Scholar]
- Hicks, B.J. Food webs in forest and pasture streams in the Waikato region, New Zealand: A study based on analyses of stable isotopes of carbon and nitrogen, and fish gut contents. N. Z. J. Mar. Freshw. Res. 1997, 31, 651–664. [Google Scholar] [CrossRef]
- Jones, J.I.; Waldron, S. Combined stable isotope and gut contents analysis of food webs in plant-dominated, shallow lakes. Freshw. Biol. 2003, 48, 1396–1407. [Google Scholar] [CrossRef]
- Sturmbauer, C.; Mark, W.; Dallinger, R. Ecophysiology of Aufwuchs-eating cichlids in Lake Tanganyika: Niche separation by trophic specialization. Environ. Biol. Fishes 1992, 35, 283–290. [Google Scholar] [CrossRef]
- Rodelli, M.R.; Gearing, J.N.; Gearing, P.J.; Marshall, N.; Sasekumar, A. Stable isotope ratio as a tracer of mangrove carbon in Malaysian ecosystems. Oecologia 1984, 61, 326–333. [Google Scholar] [CrossRef]
- Planas, M. Ecological Traits and Trophic Plasticity in The Greater Pipefish Syngnathus acus in the NW Iberian Peninsula. Biology 2022, 11, 712. [Google Scholar] [CrossRef]
- Day, E.H.; Hua, X.; Bromham, L. Is specialization an evolutionary dead end? Testing for differences in speciation, extinction and trait transition rates across diverse phylogenies of specialists and generalists. J. Evol. Biol. 2016, 29, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Liem, K.F. Adaptive Significance of Intra- and Interspecific Differences in the Feeding Repertoires of Cichlid Fishes. Am. Zool. 1980, 20, 295–314. [Google Scholar] [CrossRef]
- Robinson, B.W.; Wilson, D.S. Optimal Foraging, Specialization, and a Solution to Liem’s Paradox. Am. Nat. 1998, 151, 223–235. [Google Scholar] [CrossRef]
- Binning, S.A.; Chapman, L.J.; Cosandey-Godin, A. Specialized morphology for a generalist diet: Evidence for Liem’s Paradox in a cichlid fish. J. Fish Biol. 2009, 75, 1683–1699. [Google Scholar] [CrossRef] [PubMed]
- Golcher-Benavides, J.; Wagner, C.E. Playing out Liem’s Paradox: Opportunistic Piscivory across Lake Tanganyikan Cichlids. Am. Nat. 2019, 194, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Kruppert, S.; Summers, A.P. Fishing out a feeding paradox. Nature 2019, 571, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Nikolsky, G.V. The Ecology of Fishes; Academic Press: New York, NY, USA, 1963; 352p. [Google Scholar]
- Coad, B.W. Carps and Minnows of Iran (Families Cyprinidae and Leuciscidae). Vol. I: General Introduction and Carps (Family Cyprinidae). Available online: http://briancoad.com/Species%20Accounts/Carps%20of%20Iran%2010Sept2opt1.pdf (accessed on 20 December 2021).
- Stroud, J.T.; Losos, J.B. Ecological opportunity and adaptive radiation. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 507–532. [Google Scholar] [CrossRef]
- Losos, J.B. Integrative approaches to evolutionary ecology: Anolis lizards as model systems. Annu. Rev. Ecol. Syst. 1994, 25, 467–493. [Google Scholar] [CrossRef]
- Grant, P.R.; Grant, B.R. Unpredictable evolution in a 30-year study of Darwin’s finches. Science 2002, 296, 707–711. [Google Scholar] [CrossRef]
- Pinto, G.; Mahler, D.; Harmon, L.; Losos, J. Testing the island effect in adaptive radiation: Rates and patterns of morphological diversification in Caribbean and mainland Anolis lizards. Proc. R. Soc. B Biol. Sci. 2008, 275, 2749–2757. [Google Scholar] [CrossRef]
- Richards, E.J.; Martin, C.H. Adaptive introgression from distant Caribbean islands contributed to the diversification of a microendemic adaptive radiation of trophic specialist pupfishes. PLoS Genet. 2017, 13, e1006919. [Google Scholar] [CrossRef] [PubMed]
- Geremew, A. Taxonomic Revision, Relative Abundance, and Aspects of the Biology of Some Species of the Genus Garra, Hamilton 1922 (Pisces: Cyprinidae) in Lake Tana, Ethiopia. Doctoral Dissertation, Addis Ababa University, Addis Ababa, Ethiopia, 2007. [Google Scholar]
- Tekle-Giorgis, Y.; Yilma, H.; Dadebo, E. Feeding habits and trace metal concentrations in the muscle of lapping minnow Garra quadrimaculata (Rüppell, 1835) (Pisces: Cyprinidae) in Lake Hawassa, Ethiopia. Momona Ethiop. J. Sci. 2016, 8, 116–135. [Google Scholar] [CrossRef]
- Roberts, T.S. Garra allostoma, a new species of Cyprinid fish from highlands of the Niger basin in Cameroun. Rev. Hydrobiol. Trop. 1990, 23, 161–169. [Google Scholar]
- Timmermann, M.; Schlupp, I.; Plath, M. Shoaling behaviour in a surface-dwelling and a cave-dwelling population of a barb Garra barreimiae (Cyprinidae, Teleostei). Acta Ethologica 2004, 7, 59–64. [Google Scholar] [CrossRef]
- Savvaitova, K.A.; Shanin, A.Y.; Verigina, I.A. Speciation and species structure of false osman Schizopygopsis stoliczkai in water bodies of Pamir. Vopr. Ikhtiol. 1988, 28, 896–906. [Google Scholar]
- Grishchenko, E.V. Biology, Fishery Importance of False Osman (Schizopygopsis stoliczkai Steind. 1888) and Ways of Increase of Fish Capacity in Pamir Water Bodies. Ph.D. Thesis, Moscow State University, Moscow, Soviet Union, 1984. [Google Scholar]
- Akin, S.; Turan, H.; Kaymak, N. Does diet variation determine the digestive tract length of Capoeta banarescui Turan, Kottelat, Ekmekci and Imamoglu, 2006? J. Appl. Ichthyol. 2016, 32, 883–892. [Google Scholar] [CrossRef]
- Lammens, E.H.R.R.; Hoogenboezem, W. Diets and feeding behaviour. In Cyprinid Fishes; Winfield, I.J., Nelson, J.S., Eds.; Chapman & Hall: London, UK, 1991; pp. 353–376. [Google Scholar]








| Ecomorphs | SL Range, mm | Gut Length (n) | Diet (n) | Stable Isotopes * (n) |
|---|---|---|---|---|
| ‘generalized’ (1) | 40–162 | 80 | 80 | 30 |
| ‘stream-lined’ (2) | 71–112 | 23 | 23 | 15 |
| ‘narrow-mouth’ (3) | 47–107 | 22 | 22 | 11 |
| ‘wide-mouth’ (4) | 51–96 | 32 | 29 | 9 |
| ‘predator’ (5) | 39–193 | 26 | 26 | 14 |
| ‘thick-lipped’ (6) | 39–41 | 2 | 2 | 4 |
| Total | 185 | 182 | 83 |
| Ecomorphs | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| TA | 12.97 | 1.15 | 7.89 | 0.90 | 7.90 |
| SEA | 3.95 | 1.07 | 5.45 | 0.97 | 6.53 |
| SEAc | 4.17 | 1.34 | 6.54 | 1.29 | 8.16 |
| Ecomorphs | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | NA | 29.03 | 37.85 | 30.55 | 29.33 |
| 2 | 87.51 | NA | 48.93 | 36.50 | 40.87 |
| 3 | 35.66 | 11.54 | NA | 2.36 | 78.96 |
| 4 | 95.74 | 34.23 | 16.20 | NA | 8.48 |
| 5 | 34.06 | 9.94 | 71.31 | 1.22 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komarova, A.S.; Golubtsov, A.S.; Levin, B.A. Trophic Diversification Out of Ancestral Specialization: An Example from a Radiating African Cyprinid Fish (Genus Garra). Diversity 2022, 14, 629. https://doi.org/10.3390/d14080629
Komarova AS, Golubtsov AS, Levin BA. Trophic Diversification Out of Ancestral Specialization: An Example from a Radiating African Cyprinid Fish (Genus Garra). Diversity. 2022; 14(8):629. https://doi.org/10.3390/d14080629
Chicago/Turabian StyleKomarova, Aleksandra S., Alexander S. Golubtsov, and Boris A. Levin. 2022. "Trophic Diversification Out of Ancestral Specialization: An Example from a Radiating African Cyprinid Fish (Genus Garra)" Diversity 14, no. 8: 629. https://doi.org/10.3390/d14080629
APA StyleKomarova, A. S., Golubtsov, A. S., & Levin, B. A. (2022). Trophic Diversification Out of Ancestral Specialization: An Example from a Radiating African Cyprinid Fish (Genus Garra). Diversity, 14(8), 629. https://doi.org/10.3390/d14080629