A Quantum Cascade Laser-Based Optical Sensor for Continuous Monitoring of Environmental Methane in Dunkirk (France)
Abstract
:1. Introduction
2. Experimental Details
2.1. Experimental Setup

2.2. Selection of Methane Absorption Line for Sensitive and Selective Monitoring

2.3. Frequency Metrology and Absorption Spectrum Retrieval

2.4. Concentration Retrieval
2.5. Measurement Accuracy

2.6. Measurement Precision


3. Continuous Monitoring of CH4 at a Suburban Site in Dunkirk-France
3.1. Campaign Site Description

3.2. Complementary Measurements
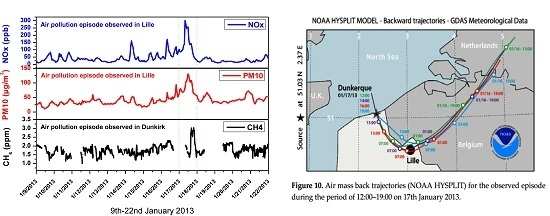
3.3. Results and Discussion






4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Battle, M.; Bender, M.; Sowers, T.; Tans, P.P.; Butler, J.H.; Elkins, J.W.; Ellis, J.T.; Conway, T.; Zhang, N.; Lang, P.; et al. Atmospheric gas concentrations over the past century measured in air from firn at the South Pole. Nature 1996, 383, 231–235. [Google Scholar] [CrossRef]
- Lelieveld, J.; Crutzen, P.J.; Dentener, F.J. Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus B 1998, 50, 128–150. [Google Scholar] [CrossRef]
- Dlugokencky, E.J.; Masarie, K.A.; Lang, P.M.; Tans, P.P. Continuing decline in the growth rate of the atmospheric methane burden. Nature 1998, 393, 447–450. [Google Scholar] [CrossRef]
- Bousquet, P.; Ciais, P.; Miller, J.B.; Dlugokencky, E.J.; Hauglustaine, D.A.; Prigent, C.; Van der Werf, G.R.; Peylin, P.; Brunke, E.-G.; Carouge, C.; et al. Contribution of anthropogenic and natural sources to atmospheric methane variability. Nature 2006, 443, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Walter, K.M.; Zimov, S.A.; Chanton, J.P.; Verbyla, D.; Chapin, F.S. Methane bubbling from Siberian thaw lakes as a positive feedback to climate warming. Nature 2006, 443, 71–75. [Google Scholar] [CrossRef] [PubMed]
- World Meteorological Organization. Guidelines for the Measurement of Methane and Nitrous Oxide and their Quality Assurance; Global Atmosphere Watch Report No. 185 (WMO/TD-No. 1478); World Meteorological Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Svanberg, S. Chemical sensing with laser spectroscopy. Sens. Actuators B Chem. 1996, 33, 1–4. [Google Scholar] [CrossRef]
- Curl, R.F.; Capasso, F.; Gmachl, C.; Kosterev, A.A.; McManus, B.; Lewicki, R.; Pusharsky, M.; Wysocki, G.; Tittel, F.K. Quantum cascade lasers in chemical physics. Chem. Phys. Lett. 2010, 487, 1–18. [Google Scholar] [CrossRef]
- Tittel, F.K.; Lewicki, R.; Lascola, R.; McWhorter, S. Emerging Infrared Laser Absorption Spectroscopic Techniques for Gas Analysis; John Wiley and Sons Inc.: New York, NY, USA, 2013; pp. 71–109. [Google Scholar]
- Grossel, A.; Zéninari, V.; Parvitte, B.; Joly, L. Courtois, D. Optimization of a compact photoacoustic quantum cascade laser spectrometer for atmospheric flux measurements: Application to the detection of methane and nitrous oxide. Appl. Phys. B 2007, 88, 483–492. [Google Scholar] [CrossRef]
- Jahjah, M.; Ren, W.; Stefanski, P.; Lewicki, R.; Zhang, J.; Jiang, W.; Tarka, J.; Tittel, F.K. A compact QCL based methane and nitrous oxide sensor for environmental and medical applications. Analyst 2014, 139, 2065–2069. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Chen, W.; Vicet, A.; Cao, Z.; Gao, X.; Nguyen-ba, T.; Jahjah, M.; Rouillard, Y.; Nahle, L.; Fischer, M. T-shape microresonator-based quartz-enhanced photoacoustic spectroscopy for ambient methane monitoring using 3.38 µm antimonide-distributed feedback laser diode. Appl. Phys. B 2013, 116, 423–428. [Google Scholar] [CrossRef]
- Lu, X.; Li, J.; Lu, H. Portable High-Precision System for CH4 Concentration Detection in Mines based on the Laser Mode-Hopping. Spectrosc. Spectr. Anal. 2015, 35, 846–849. [Google Scholar]
- Ghysels, M.; Gomez, L.; Cousin, J.; Amarouche, N.; Jost, H.; Durry, G. Spectroscopy of CH4 with a difference-frequency generation laser at 3.3 micron for atmospheric applications. Appl. Phys. B 2011, 104, 989–1000. [Google Scholar] [CrossRef]
- Mappé, I.; Joly, L.; Durry, G.; Thomas, X.; Decarpenterie, T.; Cousin, J.; Dumelie, N.; Roth, E.; Chakir, A.; Grillon, P.G. A quantum cascade laser absorption spectrometer devoted to the in situ measurements of atmospheric N2O and CH4 emission fluxes. Rev. Sci. Instrum. 2013, 84. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, L.; Tan, T.; Wang, G.; Zhang, W.; Chen, W.; Gao, X. Highly sensitive detection of methane by near-infrared laser absorption spectroscopy using a compact dense-pattern multipass cell. Sens. Actuators B Chem. 2015, 220, 1000–1005. [Google Scholar] [CrossRef]
- Crosson, E.R. A cavity ring-down analyzer for measuring atmospheric levels of methane, carbon dioxide, and water vapor. Appl. Phys. B 2008, 92, 403–408. [Google Scholar] [CrossRef]
- Gonzalez, V.R.; Magana, R.F.; Gerardo, N.O.; Sepulveda, J.A.; Martinez, C.K.; Anthony, K.W.; Baer, D.; Thalasso, F. In situ measurement of dissolved methane and carbon dioxide in freshwater ecosystems by off-axis integrated cavity output spectroscopy. Environ. Sci. Technol. 2014, 19, 11421–11428. [Google Scholar] [CrossRef] [PubMed]
- Blanquet, G.; Walrand, J.; Bouanich, J.P. Diode-laser measurements of O2-broadening coefficients in the ν3 band of CH335Cl. J. Mol. Spectrosc. 1993, 159, 137–143. [Google Scholar] [CrossRef]
- Rothman, L.S.; Jacquemart, D.; Barbe, A.; Chris Benner, D.; Birk, M.; Brown, L.R.; Carleer, M.R.; Chackerian, C.; Chance, K.; Coudert, L.H.; et al. The HITRAN 2004 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2005, 96, 139–204. [Google Scholar] [CrossRef]
- Cui, X.; Lengignon, C.; Wu, T.; Zhao, W.; Wysocki, G.; Fertein, E.; Coeur, C.; Cassez, A.; Croisé, L.; Chen, W.; et al. Photonic Sensing of the Atmosphere by absorption spectroscopy. J. Quant. Spectrosc. Rad. Transfer 2012, 113, 1300–1316. [Google Scholar] [CrossRef]
- Werle, P. Tunable diode laser absorption spectroscopy: Recent findings and novel approaches. Infrared Phys. Technol. 1996, 37, 59–66. [Google Scholar] [CrossRef]
- Werle, P.; Mücke, R.; Slemr, F. The limits of signal averaging in atmospheric trace-gas monitoring by tunable diode-laser absorption spectroscopy (TDLAS). Appl. Phys. B 1993, 57, 131–139. [Google Scholar] [CrossRef]
- Werle, P.W.; Mazzinghi, P.; D’Amato, F.; De Rosa, M.; Maurer, K.; Slemr, F. Signal processing and calibration procedures for in situ diode-laser absorption spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 1685–1705. [Google Scholar] [CrossRef] [PubMed]
- Weibring, P.; Richter, D.; Fried, A.; Walega, J.G.; Dyroff, C. Ultra-high-precision mid-IR spectrometer II: System description and spectroscopic performance. Appl. Phys. B 2006, 85, 207–218. [Google Scholar] [CrossRef]
- Cousin, J.; Chen, W.; Bigourd, D.; Fourmentin, M.; Kassi, S. Telecom-grade fiber laser-based difference-frequency generation and ppb-level detection of benzene vapor in air around 3 μm. Appl. Phys. B 2009, 97, 919–929. [Google Scholar] [CrossRef]
- Draxler, R.R.; Rolph, G.D. HYSPLIT (HYbrid Single-Particle Lagrangian Integrated Trajectory). NOAA Air Resources Laboratory: College Park, MD, USA. Available online: http://www.arl.noaa.gov/HYSPLIT.php (accessed on 11 December 2015).
- ATMO Nord–Pas-de-Calais. Available online: http://www.atmo-npdc.fr/ (accessed on 25 January 2016).
- AP-42. Compilation of Air Pollutant Emission Factors, 5th ed.Environmental Protection Agency: Washington, DC, USA, 1995.
- Nitrogen Oxides (NOx). Why and How They Are Controlled; Technical Report EPA-456/F-99–006R; Environmental Protection Agency: Washington, DC, USA, 1999.
- McKain, K.; Down, A.; Raciti, S.M.; Budney, J.; Hutyra, L.R.; Floerchinger, C.; Herndon, S.C.; Nehrkorn, T.; Zahniser, M.S.; Jackson, R.B.; et al. Methane emissions from natural gas infrastructure and use in the urban region of Boston, Massachusetts. PNAS 2015, 112, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maamary, R.; Cui, X.; Fertein, E.; Augustin, P.; Fourmentin, M.; Dewaele, D.; Cazier, F.; Guinet, L.; Chen, W. A Quantum Cascade Laser-Based Optical Sensor for Continuous Monitoring of Environmental Methane in Dunkirk (France). Sensors 2016, 16, 224. https://doi.org/10.3390/s16020224
Maamary R, Cui X, Fertein E, Augustin P, Fourmentin M, Dewaele D, Cazier F, Guinet L, Chen W. A Quantum Cascade Laser-Based Optical Sensor for Continuous Monitoring of Environmental Methane in Dunkirk (France). Sensors. 2016; 16(2):224. https://doi.org/10.3390/s16020224
Chicago/Turabian StyleMaamary, Rabih, Xiaojuan Cui, Eric Fertein, Patrick Augustin, Marc Fourmentin, Dorothée Dewaele, Fabrice Cazier, Laurence Guinet, and Weidong Chen. 2016. "A Quantum Cascade Laser-Based Optical Sensor for Continuous Monitoring of Environmental Methane in Dunkirk (France)" Sensors 16, no. 2: 224. https://doi.org/10.3390/s16020224
APA StyleMaamary, R., Cui, X., Fertein, E., Augustin, P., Fourmentin, M., Dewaele, D., Cazier, F., Guinet, L., & Chen, W. (2016). A Quantum Cascade Laser-Based Optical Sensor for Continuous Monitoring of Environmental Methane in Dunkirk (France). Sensors, 16(2), 224. https://doi.org/10.3390/s16020224