Nitrogen-Doped Carbon Dots as A New Substrate for Sensitive Glucose Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Preparation and Purification of the N-Doped CDs
2.3. Preparation and Purification of the Pure Glucose-Based CDs
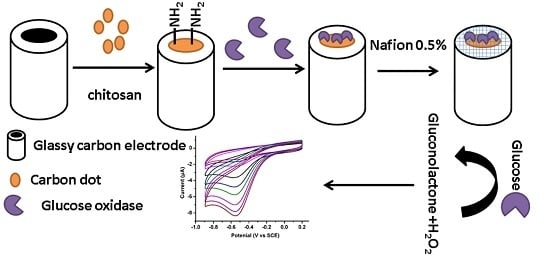
2.4. Preparation of N-Doped CDs/Chitosan/GOx Modified GCE
2.5. Detection of Glucose with N-Doped CDs/Chitosan/GOx Modified GCE
3. Results and Discussion
3.1. Characterization of N-Doped CDs
3.2. Electrocatalysis of O2 Reduction at N-Doped CDs Modified GCE
3.3. Detection of Glucose Based on the N-Doped CDs Modified GCE
3.4. Analytical Performance
3.5. Interference Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CDs | Carbon Dots |
| GOx | Glucose oxidase |
| N-doped | Nitrogen-doped |
References
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Meziani, M.J.; Liu, J.-H.; Liu, Y.; Chen, M.; et al. Carbon dots as nontoxic and high-performance fluorescence imaging agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lin, J. Defect-related luminescent materials: Synthesis, emission properties and applications. Chem. Soc. Rev. 2012, 41, 7938–7961. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, M.; Liu, Y.; Feng, X.-Z.; Yin, X.-B.; He, X.-W.; Zhang, Y.-K. Nitrogen-doped carbon dots: A facile and general preparation method, photoluminescence investigation, and imaging applications. Chem. Eur. J. 2013, 19, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Yu, P.; Wen, X.; Toh, Y.-R.; Tang, J. Temperature-dependent fluorescence in carbon dots. J. Phys. Chem. C 2012, 116, 25552–25557. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Wang, X.; Wang, Z.; Zhang, C.; He, M.; Wang, K.; Chen, F.; Li, Z.; Shen, G.; et al. Light-triggered theranostics based on photosensitizer-conjugated carbon dots for simultaneous enhanced-fluorescence imaging and photodynamic therapy. Adv. Mater. 2012, 24, 5104–5110. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, D.; Liu, Y.; Xu, X.; Xiong, C. Nitrogen-doped carbon dots from plant cytoplasm as selective and sensitive fluorescent probes for detecting p-nitroaniline in both aqueous and soil systems. Analyst 2015, 140, 1428–1431. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, C.; Chen, W.; Weng, S.; Liu, A.; Liu, Q.; Zheng, Z.; Lin, J.; Lin, X. Sensitive electrochemical immunoassay of metallothionein-3 based on K3[Fe(CN)6] as a redox-active signal and c-dots/nafion film for antibody immobilization. Analyst 2013, 138, 7341–7346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, X.; Zeng, F.; Zheng, F.; Wu, S. Carbon-dot-based ratiometric fluorescent sensor for detecting hydrogen sulfide in aqueous media and inside live cells. Chem. Commun. 2013, 49, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Moschou, E.A.; Sharma, B.V.; Deo, S.K.; Daunert, S. Fluorescence glucose detection: Advances toward the ideal in vivo biosensor. J. Fluoresc. 2004, 14, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New directions in screen printed electroanalytical sensors: An overview of recent developments. Analyst 2011, 136, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Turner, A.P.F. Glucose-oxidase-an ideal enzyme. Biosens. Bioelectron. 1992, 7, 165–185. [Google Scholar] [CrossRef]
- Nayak, P.; Santhosh, P.N.; Ramaprabhu, S. Synthesis of au-mwcnt-graphene hybrid composite for the rapid detection of H2O2 and glucose. RSC Adv. 2014, 4, 41670–41677. [Google Scholar] [CrossRef]
- Razmi, H.; Mohammad-Rezaei, R. Graphene quantum dots as a new substrate for immobilization and direct electrochemistry of glucose oxidase: Application to sensitive glucose determination. Biosens. Bioelectron. 2013, 41, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Lee, G.-J.; Ryu, J.-H.; Park, K.; Park, H.-K. A flexible, nonenzymatic glucose biosensor based on ni-coordinated, vertically aligned carbon nanotube arrays. RSC Adv. 2014, 4, 48310–48316. [Google Scholar] [CrossRef]
- Kiani, M.A.; Tehrani, M.A.; Sayahi, H. Reusable and robust high sensitive non-enzymatic glucose sensor based on Ni(OH)2 nanoparticles. Anal. Chim. Acta 2014, 839, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, A.P.; Chang, Y.-J.; Chen, S.-M. Amperometric glucose sensor based on glucose oxidase immobilized on gelatin-multiwalled carbon nanotube modified glassy carbon electrode. Bioelectrochemistry 2011, 80, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Ceja-Fdez, A.; Lopez-Luke, T.; Torres-Castro, A.; Wheeler, D.A.; Zhang, J.Z.; De la Rosa, E. Glucose detection using sers with multi-branched gold nanostructures in aqueous medium. RSC Adv. 2014, 4, 59233–59241. [Google Scholar] [CrossRef]
- Qiu, K.; Chen, X.; Ci, S.; Li, W.; Bo, Z.; Cen, K.; Wen, Z. Facile preparation of nickel nanoparticle-modified carbon nanotubes with application as a nonenzymatic electrochemical glucose sensor. Anal. Lett. 2016, 49, 568–578. [Google Scholar] [CrossRef]
- Lin, K.C.; Hung, Y.T.; Chen, S.M. Facile preparation of a highly sensitive nonenzymatic glucose sensor based on multiwalled carbon nanotubes decorated with electrodeposited metals. RSC Adv. 2015, 5, 2806–2812. [Google Scholar] [CrossRef]
- Lv, C.; Di, W.; Liu, Z.; Zheng, K.; Qin, W. Luminescent cepo4: Tb colloids for H2O2 and glucose sensing. Analyst 2014, 139, 4547–4555. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Zhao, X.J.; Yang, X.X.; Li, Y.F. A nanosized metal-organic framework of Fe-MIL-88NH2 as a novel peroxidase mimic used for colorimetric detection of glucose. Analyst 2013, 138, 4526–4531. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.-Y.; Choi, Y.-B.; Kim, H.-H. Disposable non-enzymatic glucose sensors using screen-printed nickel/carbon composites on indium tin oxide electrodes. Sensors 2015, 15, 31083–31091. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, C.; Abdi, M.M.; Mathew, A.P.; Jonoobi, M.; Oksman, K.; Rezayi, M. Synergy effect of nanocrystalline cellulose for the biosensing detection of glucose. Sensors 2015, 15, 24681–24697. [Google Scholar] [CrossRef] [PubMed]
- Marie, M.; Mandal, S.; Manasreh, O. An electrochemical glucose sensor based on zinc oxide nanorods. Sensors 2015, 15, 18714–18723. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Jian, G.; Lei, J.; Hu, Z.; Ju, H. A glucose biosensor based on direct electrochemistry of glucose oxidase immobilized on nitrogen-doped carbon nanotubes. Biosens. Bioelectron. 2009, 25, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Vashist, S.K.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.-S. Mediatorless amperometric glucose biosensing using 3-aminopropyltriethoxysilane-functionalized graphene. Talanta 2012, 99, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Sorlier, P.; Denuziere, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, X.; Zhang, J.; Boey, F.; Zhang, H. Direct electrochemical reduction of single-layer graphene oxide and subsequent functionalization with glucose oxidase. J. Phys. Chem. C 2009, 113, 14071–14075. [Google Scholar] [CrossRef]
- Chen, L.; Gorski, W. Bioinorganic composites for enzyme electrodes. Anal. Chem. 2001, 73, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, W.; Zhang, Q.; Meng, Z.; Jia, X.; Xi, K. Synthesis of fluorescent carbon nanoparticles from polyacrylamide for fast cellular endocytosis. RSC Adv. 2013, 3, 15589–15591. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Yang, Y.H.; Liu, Y.L.; Shen, G.L.; Yu, R.Q. Platinum nanoparticles-doped sol-gel/carbon nanotubes composite electrochemical sensors and biosensors. Biosens. Bioelectron. 2006, 21, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Mai, Z.; Zou, X.; Cai, P.; Mo, J. Glucose biosensors based on platinum nanoparticles-deposited carbon nanotubes in sol-gel chitosan/silica hybrid. Talanta 2008, 74, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, D.; Zhao, T.; Zeng, B. Development of amperometric glucose biosensor through immobilizing enzyme in a pt nanoparticles/mesoporous carbon matrix. Talanta 2008, 74, 1586–1591. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Zhou, F.; Gu, J.; Shu, C.; Xi, K.; Jia, X. Nitrogen-Doped Carbon Dots as A New Substrate for Sensitive Glucose Determination. Sensors 2016, 16, 630. https://doi.org/10.3390/s16050630
Ji H, Zhou F, Gu J, Shu C, Xi K, Jia X. Nitrogen-Doped Carbon Dots as A New Substrate for Sensitive Glucose Determination. Sensors. 2016; 16(5):630. https://doi.org/10.3390/s16050630
Chicago/Turabian StyleJi, Hanxu, Feng Zhou, Jiangjiang Gu, Chen Shu, Kai Xi, and Xudong Jia. 2016. "Nitrogen-Doped Carbon Dots as A New Substrate for Sensitive Glucose Determination" Sensors 16, no. 5: 630. https://doi.org/10.3390/s16050630
APA StyleJi, H., Zhou, F., Gu, J., Shu, C., Xi, K., & Jia, X. (2016). Nitrogen-Doped Carbon Dots as A New Substrate for Sensitive Glucose Determination. Sensors, 16(5), 630. https://doi.org/10.3390/s16050630