Silica-gel Particles Loaded with an Ionic Liquid for Separation of Zr(IV) Prior to Its Determination by ICP-OES
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
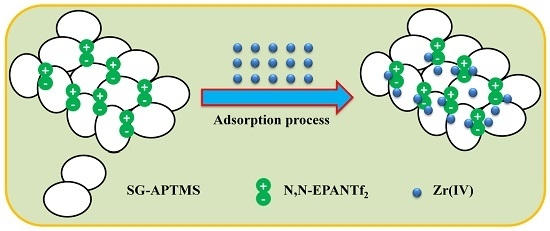
2.2. Preparation of the New Adsorbent Based on Silica Gel
2.2.1. Preparation of N,N-EPANTf2 Ionic Iiquid
2.2.2. Activation of SG
2.2.3. Synthesis of SG-APTMS
2.2.4. Synthesis of SG-APTMS-N,N-EPANTf2
2.3. Batch Procedure
2.4. Instrumentation
3. Results and Discussion
3.1. Characterization of the SG-APTMS-N,N-EPANTf2
3.1.1. Surface Coverage Value of the SG-APTMS-N,N-EPANTf2 Phase
3.1.2. FT-IR Analysis
3.1.3. SEM Analysis
3.2. Batch Method
3.2.1. Effect of pH and Selectivity Study
3.2.2. Determination of Zr(IV) Adsorption Capacity
3.3. Adsorption Isotherm Models
3.4. Effect of Contact Time
Kinetic Models
3.5. Performance of the Method in Analytical Applications
3.5.1. Effect of Interfering Ions
3.5.2. Real Sample Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vidal, L.; Riekkola, M.-L.; Canals, A. Ionic liquid-modified materials for solid-phase extraction and separation: A review. Anal. Chim. Acta 2012, 715, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Khatun, S.; Castner, E.W., Jr. Communication: Unusual structure and transport in ionic liquid-hexane mixtures. J. Chem. Phys. 2015, 142, 121101. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Bi, W.; Tian, M.; Row, K. H. Application of ionic liquid for extraction and separation of bioactive compounds from plants. J. Chromatogr. B 2012, 904, 1–21. [Google Scholar] [CrossRef] [PubMed]
- García, S.; García, J.; Larriba, M.; Casas, A.; Rodríguez, F. Liquid-liquid extraction of toluene from heptane by {[4bmpy][Tf2N] + [emim][CHF2CF2SO3]} ionic liquid mixed solvents. Fluid Phase Equilib. 2013, 337, 47–52. [Google Scholar] [CrossRef]
- Marwani, H.M. Selective separation and determination of Lead based on silica gel developed by surface adsorbed new hydrophobic ionic liquid. J. Dispersion Sci. Technol. 2013, 34, 117–124. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Al-Bishri, H.M. Supported hydrophobic ionic liquid on nano-silica for adsorption of lead. Chem. Eng. J. 2011, 166, 157–167. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Luo, F.; Chen, J. The preparation of sol-gel materials doped with ionic liquids and trialkyl phosphine oxides for yttrium(III) uptake. Anal. Chim. Acta 2007, 604, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F.; Poole, S.K. Ionic liquid stationary phases for gas chromatography. J. Sep. Sci. 2011, 34, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Jiang, S.; Liu, X. N-Methylimidazolium anion-exchange stationary phase for high performance liquid chromatography. J. Chromatogr. A 2006, 1103, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Borissova, M.; Vaher, M.; Koel, M.; Kaljurand, M. Capillary zone electrophoresis on chemically bonded imidazolium based salts. J. Chromatogr. A 2007, 1160, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Crank, J.A.; Armstrong, D.W. Towards a second generation of ionic liquids matrices (ILMs) for MALDI-MS of peptides, proteins and carbohydrates. J. Am. Soc. Mass. Spectrom. 2009, 20, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, T. Electrochemical aspects of ionic-liquid/water two-phase systems. Anal. Chem. 2007, 79, 6442–6449. [Google Scholar] [CrossRef]
- Forzani, E.S.; Lu, D.; Leright, M.J.; Aguilar, A.D.; Tsow, F.; Iglesias, R.A.; Zhang, Q.; Lu, J.; Li, J.; Tao, N. A hybrid electrochemical–colorimetric sensing platform for detection of explosives. J. Am. Chem. Soc. 2009, 131, 1390–1391. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.D.; Oliveira, D. Fluorescence determination of enantiomeric composition of pharmaceuticals via use of ionic liquid that serves as both solvent and chiral selector. Anal. Biochem. 2006, 356, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Marwani, H.M. Spectroscopic evaluation of chiral and achiral fluorescent ionic liquids. Cent. Eur. J. Chem. 2010, 8, 946–952. [Google Scholar] [CrossRef]
- Bwambok, D.K.; Marwani, H.M.; Fernand, V.E.; Fakayode, S.O.; Lowry, M.; Negulescu, I.; Strongin, R.M.; Warner, I.M. Synthesis and characterization of novel chiral ionic liquids and investigation of their enantiomeric recognition properties. Chirality 2008, 20, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and composition of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, S.; Pan, L.; Li, J.; Liu, F.; Liu, H. Spectroscopic studies on the interactions between imidazolium chloride ionic liquids and bovine serum albumin. Spectrochim. Acta Part A 2013, 104, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Panda, A.K. Physico-chemical studies on ionic liquid microemulsion: Phase manifestation, formation dynamics, size, viscosity, percolation of electrical conductance and spectroscopic investigations of 1-butyl-3-methyl imidazolium methanesulfonate+water/Tween 20+ n-pentanol/n-heptane pseudoternary system. Colloids Surf. A 2013, 419, 113–124. [Google Scholar]
- Sebastiao, E.; Cook, C.; Hu, A.; Murugesu, M. Recent developments in the fields of energetic ionic liquids. J. Mater. Chem. A 2014, 2, 8153–8173. [Google Scholar] [CrossRef]
- Chhotaray, P.K.; Gardas, R.L. Thermophysical properties of ammonium and hydroxylammonium protic ionic liquids. J. Chem. Thermodyn. 2014, 72, 117–124. [Google Scholar] [CrossRef]
- Sun, X.; Peng, B.; Ji, Y.; Chen, J.; Li, D. The solid-liquid extraction of yttrium from rare earths by solvent (ionic liquid) impreganated resin couples with complexing method. Sep. Purif. Technol. 2008, 63, 61–68. [Google Scholar] [CrossRef]
- Pavagadhi, S.; Basheer, C.; Balasubramanian, R. Application of ionic-liquid supported cloud point extraction for the determination of microcystin-leucine-arginine in natural waters. Anal. Chim. Acta 2011, 686, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Berton, P.; Monasterio, R.P.; Wuilloud, R.G. Selective extraction and determination of vitamin B12 in urine by ionic liquid-based aqueous two-phase system prior to high-performance liquid chromatography. Talanta 2012, 97, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Corderí, S.; González, E.J.; Calvar, N.; Domínguez, Á. Application of [HMim] [NTf2], [HMim] [TfO], and [BMim] [TfO] ionic liquids on the extraction of toluene from alkanes: Effect of the anion and the alkyl chain length of the cation on the LLE. J. Chem. Thermodyn. 2012, 53, 60–66. [Google Scholar] [CrossRef]
- Rodrigues, G.D.; de Lemos, L.R.; da Silva, L.H.M.; da Silva, M.C.H. Application of hydrophobic extractant in aqueous two-phase systems for selective extraction of cobalt, nickel and cadmium. J. Chromatogr. A 2013, 1279, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Faghihian, H.; Kabiri-Tadi, M. A novel solid-phase extraction method for separation and preconcentration of zirconium. Microchim. Acta 2010, 168, 147–152. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Yilmaz, E.; Habila, M.; Soylak, M. Solid phase extraction of metal ions in environmental samples on 1-(2-pyridylazo)-2-naphthol impregnated activated carbon cloth. Ecotox. Environ. Safe. 2015, 112, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, A.; Saber-Tehrani, M.; Bagheri, H.; Madrakian, T. Flame atomic absorption spectrometric determination of trace amounts of Pb(II) and Cr(III) in biological, food and environmental samples after preconcentration by modified nano-alumina. Microchim. Acta 2011, 172, 125–136. [Google Scholar] [CrossRef]
- Ngeontae, W.; Aeungmaitrepirom, W.; Tuntulani, T. Chemically modified silica gel with aminothioamidoantraquinone for solid phase extraction and preconcentration of Pb(II), Cu(II), Ni(II), Co(II), and Cd(II). Talanta 2007, 71, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Lertlapwasin, R.; Bhawawet, N.; Imyim, A.; Fuangswasdi, S. Ionic liquid extraction of heavy metal ion by 2-aminothiophenol in 1-butyl-3-methylimidazolium hexafluorophosphate and their association constants. Sep. Purif. Technol. 2010, 72, 70–76. [Google Scholar] [CrossRef]
- Jie, C.; Zaijun, L.; Ming, L. Spectrophotometric determination of ultra-trace uranium(VI) in seawater after extractive preconcentration with ionic liquid and dimethylphenylazosalicylfluorone. Int. J. Environ. Anal. Chem. 2008, 88, 583–590. [Google Scholar] [CrossRef]
- Sun, X.Q.; Peng, B.; Chen, J.; Li, D.Q.; Luo, F. An effective method for enhancing metal-ions selectivity of ionic liquid-based extraction system: Adding water-soluble complexing agent. Talanta 2008, 74, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ji, Y.; Guo, L.; Chen, J.; Li, D. A novel ammonium ionic liquid based extraction strategy for separating scandium from yttrium and lanthanides. Sep. Purif. Technol. 2011, 81, 25–30. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Collado-González, M.; Trigo, R.; Díaz Baños, F.G.; Víllora, G. Experimental measurements of octanol-water partition coefficients of ionic liquids. J. Adv. Chem. Eng. 2015, 5, 1000133. [Google Scholar]
- Azimova, M.A.; Morton, S.A.; Frymier, P.D. Toxicity of imidazolium-based ionic liquids to industrial wastewater treatment bacteria. In Proceedings of the IEEE 32nd Annual Northeast Bioengineering Conference, Easton, PA, USA, 1–2 April 2006; pp. 11–12.
- Sun, X.; Wu, D.; Chen, J.; Li, D. Separation of scandium(III) from lanthanides(III) with room temperature ionic liquid based extraction containing Cyanex 925. J. Chem. Technol. Biotechnol. 2007, 82, 267–272. [Google Scholar] [CrossRef]
- Chen, M.-L.; Zhao, Y.-N.; Zhang, D.-W.; Tian, Y.; Wang, J.-H. The immobilization of hydrophilic ionic liquid for Cr(VI) retention and chromium speciation. J. Anal. Atom. Spectrom. 2010, 25, 1688–1694. [Google Scholar] [CrossRef]
- Ayata, S.; Bozkurt, S.S.; Ocakoglu, K. Separation and preconcentration of Pb(II) using ionic liquid-modified silica and its determination by flame atomic absorption spectroscopy. Talanta 2011, 84, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Kooshki, M.; Shams, E. Selective response of dopamine in the presence of ascorbic acid on carbon paste electrode modified with titanium phosphates silica gel. Anal. Chim. Acta 2007, 587, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Suneesh, A.S.; Syamala, K.V.; Venkatesan, K.A.; Antony, M.P.; Vasudeva Rao, P.R. Diglycolamic acid modified silica gel for the separation of hazardous trivalent metal ions from aqueous solution. J. Colloid Interface Sci. 2015, 438, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Hard and soft acid and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Marwani, H.M. Exploring spectroscopic and physicochemical properties of new fluorescent ionic liquids. J. Fluoresc. 2013, 23, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Marwani, H.M.; Alsafrani, A.E. New solid phase extractor based on ionic liquid functionalized silica gel surface for selective separation and determination of lanthanum. J. Anal. Sci. Technol. 2013, 4. [Google Scholar] [CrossRef]
- Han, D.-M.; Fang, G.-Z.; Yan, X.-P. Preparation and evaluation of a molecularly imprinted sol-gel material for on-line solid phase extraction coupled with high performance liquid chromatography for the determination of trace pentachlorophenol in water samples. J. Chromatogr. A 2005, 1100, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Zolfonoun, E.; Monji, A.B.; Taghizadeh, M.; Ahmadi, S.J. Selective and direct sorption of zirconium from acidic leach liquor of zircon concentrate by rice bran. Miner. Eng. 2010, 23, 755–756. [Google Scholar] [CrossRef]
- Favre-Réguillon, A.; Fiaty, K.; Laurent, P.; Poriel, L.; Pellet-Rostaing, S.; Lemaire, M. Solid/liquid extraction of zirconium and hafnium in hydrochloric acid aqueous solution with anion exchange resin-kinetic study and equilibrium analysis. Ind. Eng. Chem. Res. 2007, 46, 1286–1291. [Google Scholar] [CrossRef]
- Akhtar, K.; Akhtar, M.W.; Khalid, A.M. Removal and recovery of zirconium from its aqueous solution by Candida tropicalis. J. Hazard. Mater. 2008, 156, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- McKay, G.; Blair, H.S.; Gardner, J.R. Adsorption of dyes on chitin. I. Eqiulibrium studies. J. Appl. Polym. Sci. 1982, 27, 3043–3057. [Google Scholar] [CrossRef]
- Rao, M.M.; Reddy, D.H.K.K.; Venkateswarlu, P.; Seshaiah, K. Removal of mercury from aqueous solutions using activated carbon prepared from agricultural by products/waste. J. Environ. Manage. 2009, 90, 634–643. [Google Scholar] [CrossRef] [PubMed]








| Metal Ion | Concentration (mg·L−1) | qe (mg·g−1) | Kd (mL·g−1) |
|---|---|---|---|
| Zr(IV) | 2.00 | 2.49 | 289.45 × 103 |
| Se(IV) | 2.00 | 0.31 | 176.94 |
| Cr(III) | 2.00 | 0.33 | 186.86 |
| Au(III) | 2.00 | 0.30 | 168.84 |
| Pb(II) | 2.00 | 0.17 | 92.12 |
| Hg(II) | 2.00 | 0.16 | 86.90 |
| Cu(II) | 2.00 | 0.12 | 64.41 |
| Co(II) | 2.00 | 0.01 | 6.28 |
| Coexisting Ions | Concentration (mg·L−1) | Extraction of Zr(IV) (%) |
|---|---|---|
| Na+, K+, NH4+ | 3000 | 98.37 |
| Ca2+, Mg2+ | 3000 | 97.04 |
| Cd2+ | 500 | 97.58 |
| Cu2+ | 500 | 96.17 |
| Pb2+ | 500 | 96.90 |
| Zn2+ | 600 | 95.24 |
| Fe3+ | 300 | 93.88 |
| Al3+ | 400 | 95.33 |
| Cr3+ | 400 | 94.45 |
| Cl−, F−, NO3− | 7000 | 98.57 |
| CO32−, SO42− | 6000 | 97.39 |
| PO43− | 5000 | 98.84 |
| Samples | Added (mg·L−1) | Unadsorbed (mg·L−1) | Extraction (%) |
|---|---|---|---|
| Tap water | 1 | 0.01 (±0.031) | 98.79 |
| 5 | 0.14 (±0.166) | 97.27 | |
| 25 | 0.69 (±0.317) | 97.26 | |
| Lake water | 1 | 0.01 (±0.024) | 98.53 |
| 5 | 0.17 (±0.133) | 96.67 | |
| 25 | 0.91 (±0.253) | 96.35 | |
| Seawater | 1 | 0.02 (±0.079) | 97.86 |
| 5 | 0.23 (±0.182) | 95.49 | |
| 25 | 1.38 (±0.421) | 94.47 | |
| Drinking water | 1 | 0.01 (±0.015) | 98.96 |
| 5 | 0.11 (±0.109) | 97.76 | |
| 25 | 0.47 (±0.157) | 98.12 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marwani, H.M.; Alsafrani, A.E.; Asiri, A.M.; Rahman, M.M. Silica-gel Particles Loaded with an Ionic Liquid for Separation of Zr(IV) Prior to Its Determination by ICP-OES. Sensors 2016, 16, 1001. https://doi.org/10.3390/s16071001
Marwani HM, Alsafrani AE, Asiri AM, Rahman MM. Silica-gel Particles Loaded with an Ionic Liquid for Separation of Zr(IV) Prior to Its Determination by ICP-OES. Sensors. 2016; 16(7):1001. https://doi.org/10.3390/s16071001
Chicago/Turabian StyleMarwani, Hadi M., Amjad E. Alsafrani, Abdullah M. Asiri, and Mohammed M. Rahman. 2016. "Silica-gel Particles Loaded with an Ionic Liquid for Separation of Zr(IV) Prior to Its Determination by ICP-OES" Sensors 16, no. 7: 1001. https://doi.org/10.3390/s16071001
APA StyleMarwani, H. M., Alsafrani, A. E., Asiri, A. M., & Rahman, M. M. (2016). Silica-gel Particles Loaded with an Ionic Liquid for Separation of Zr(IV) Prior to Its Determination by ICP-OES. Sensors, 16(7), 1001. https://doi.org/10.3390/s16071001