A Ratiometric Fluorescent Sensor for Cd2+ Based on Internal Charge Transfer
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials and Instrumentation
2.2. Molecular Synthesis
2.3. Sample Preparation and Spectral Measurements
3. Results and Discussion
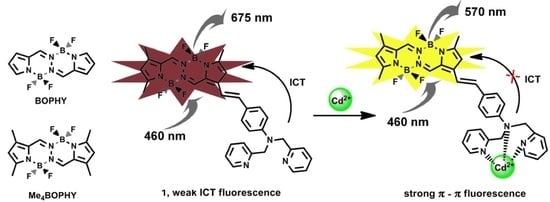
3.1. Spectral Change of 1 Upon Titration with Cd2+
3.2. Sensing Mechanism and Job’s Plot
3.3. Sensing Selectivity
3.4. Fast Sensor Response
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced Cancers in Animals and in Humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: http://www.who.int/water_sanitation_health/publications/drinking-water-quality-guidelines-4-including-1st-addendum/en/ (accessed on 1 November 2017).
- Wen, X.D.; Yang, Q.L.; Yan, Z.D.; Deng, Q.W. Determination of cadmium and copper in water and food samples by dispersive liquid–liquid microextraction combined with UV–vis spectrophotometry. Microchem. J. 2011, 97, 249–254. [Google Scholar] [CrossRef]
- Manzoori, J.L.; Bavili-Tabrizi, A. Cloud point preconcentration and flame atomic absorption spectrometric determination of Cd and Pb in human hair. Anal. Chim. Acta 2002, 470, 215–221. [Google Scholar] [CrossRef]
- Rao, K.S.; Balaji, T.; Rao, T.P.; Babu, Y.; Naidu, G.R.K. Determination of iron, cobalt, nickel, manganese, zinc, copper, cadmium and lead in human hair by inductively coupled plasma-atomic emission spectrometry. Spectrochim. Acta Part B 2002, 57, 1333–1338. [Google Scholar]
- Xue, L.; Liu, C.; Jiang, H. Highly Sensitive and Selective Fluorescent Sensor for Distinguishing Cadmium from Zinc Ions in Aqueous Media. Org. Lett. 2009, 11, 1655–1658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, R.F.; Xing, S.K.; Liu, X.M.; Hu, T.L.; Bu, X.H. A Highly Selective On/Off Fluorescence Sensor for Cadmium(II). Inorg. Chem. 2011, 50, 10041–10046. [Google Scholar] [CrossRef] [PubMed]
- Gunnlaugsson, T.; Lee, T.C.; Parkesh, R. Highly selective fluorescent chemosensors for cadmium in water. Tetrahedron 2004, 60, 11239–11249. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wang, R.J.; Fan, C.B.; Liu, G.; Pu, S.Z. A highly selective fluorescent sensor for Cd2+ based on a new diarylethene with a 1,8-naphthyridine unit. Dyes Pigments 2017, 139, 208–217. [Google Scholar] [CrossRef]
- Khani, R.; Ghiamati, E.; Boroujerdi, R.; Rezaeifard, A.; Zaryabi, M.H. A new and highly selective turn-on fluorescent sensor with fast response time for the monitoring of cadmium ions in cosmetic, and health product samples. Spectrochim. Acta Part A 2016, 163, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.B. Highly selective detection of Zn2+ and Cd2+ with a simple amino-terpyridine compound in solution and solid state. J. Chem. Sci. 2016, 128, 133–139. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Li, P.X.; Shi, Z.H.; Tang, X.L.; Chen, C.Y.; Liu, W.S. A Highly Selective Fluorescent Sensor for Distinguishing Cadmium from Zinc Ions Based on a Quinoline Platform. Inorg. Chem. 2012, 51, 9226–9231. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Das, D.K. A New Highly Sensitive and Selective Fluorescent Cadmium Sensor. J. Fluoresc. 2012, 22, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xiao, Y.; Qian, X.H. A highly selective Cd2+ sensor of naphthyridine: fluorescent enhancement and red-shift by the synergistic action of forming binuclear complex. Tetrahedron Lett. 2008, 49, 3380–3384. [Google Scholar] [CrossRef]
- Mameli, M.; Aragoni, M.C.; Arca, M.; Caltagirone, C.; Demartin, F.; Farruggia, G.; De Filippo, G.; Devillanova, F.A.; Garau, A.; Isaia, F.; et al. A Selective, Nontoxic, OFF–ON Fluorescent Molecular Sensor Based on 8-Hydroxyquinoline for Probing Cd2+ in Living Cells. Chem. Eur. J. 2010, 16, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiao, Q.L.; Zhao, M.; Yin, W.T.; Miao, L.; Wang, L.Q.; Xu, Z.C. Cd2+-triggered amide tautomerization produces a highly Cd2+-selective fluorescent sensor across a wide pH range. Dyes Pigments 2016, 133, 339–344. [Google Scholar] [CrossRef]
- Lu, C.L.; Xu, Z.C.; Cui, J.N.; Zhang, R.; Qian, X.H. Ratiometric and Highly Selective Fluorescent Sensor for Cadmium under Physiological pH Range: A New Strategy to Discriminate Cadmium from Zinc. J. Org. Chem. 2007, 72, 3554–3557. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Li, G.P.; Liu, Q.; Wang, H.H.; Liu, C.; Ding, X.L.; He, S.G.; Jiang, H. Ratiometric Fluorescent Sensor Based on Inhibition of Resonance for Detection of Cadmium in Aqueous Solution and Living Cells. Inorg. Chem. 2011, 50, 3680–3690. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Desaki, M.; Ojida, A.; Iyoshi, S.; Hirayama, T.; Hamachi, I.; Yamamoto, Y. Fluorescence Imaging of Intracellular Cadmium Using a Dual-Excitation Ratiometric Chemosensor. J. Am. Chem. Soc. 2008, 130, 12564–12565. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.Y.; Chen, P.H.; Chang, C.L.; Yang, D.M. Live-Cell Dynamic Sensing of Cd2+ with a FRET-Based Indicator. PLoS ONE 2013, 8, e65853. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, H.L.; Bunes, B.R.; Wu, N.; Xu, M.; Yang, X.M.; Yu, L.; Zang, L. Trace Detection of RDX, HMX and PETN Explosives Using a Fluorescence Spot Sensor. Sci. Rep. 2016, 6, 25015. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Han, J.M.; Wang, C.; Yang, X.M.; Pei, J.; Zang, L. Fluorescence Ratiometric Sensor for Trace Vapor Detection of Hydrogen Peroxide. ACS Appl. Mater. Interfaces 2014, 6, 8708–8714. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, M.; Johnsson, K. Localizable and Highly Sensitive Calcium Indicator Based on a BODIPY Fluorophore. Anal. Chem. 2010, 82, 6472–6479. [Google Scholar] [CrossRef] [PubMed]
- Atilgan, S.; Kutuk, I.; Ozdemir, T. A near IR distyryl BODIPY-based ratiometric fluorescent chemosensor for Hg(II). Tetrahedron Lett. 2010, 51, 892–894. [Google Scholar] [CrossRef]
- Qi, X.; Jun, E.J.; Xu, L.; Kim, S.J.; Joong Hong, J.S.; Yoon, Y.J.; Yoon, J. New BODIPY Derivatives as OFF−ON Fluorescent Chemosensor and Fluorescent Chemodosimeter for Cu2+: Cooperative Selectivity Enhancement toward Cu2+. J. Org. Chem. 2006, 71, 2881–2884. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Peng, X.J.; Guo, B.C.; Fan, J.L.; Zhang, Z.C.; Wang, J.Y.; Cui, A.J.; Gao, Y.L. Boron dipyrromethene fluorophore based fluorescence sensor for the selective imaging of Zn (II) in living cells. Org. Biomol. Chem. 2005, 3, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, K.; Li, S.; Song, T.; Han, Y.F.; Li, X. A highly sensitive and selective fluorescent chemosensor for Pb2+ ions in an aqueous solution. Dalton Trans. 2013, 42, 3854–3859. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.Y.; Xu, Y.F.; Zhang, S.Y.; Zhu, W.P.; Qian, X.H.; Duan, L.P. A Highly Sensitive and Selective OFF-ON Fluorescent Sensor for Cadmium in Aqueous Solution and Living Cell. J. Am. Chem. Soc. 2008, 130, 16160–16161. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.J.; Du, J.J.; Fan, J.L.; Wang, J.Y.; Wu, Y.K.; Zhao, J.Z.; Sun, S.G.; Xu, T. A Selective Fluorescent Sensor for Imaging Cd2+ in Living Cells. J. Am. Chem. Soc. 2007, 129, 1500–1501. [Google Scholar] [CrossRef] [PubMed]
- Tamgho, I.S.; Hasheminasab, A.; Engle, J.T.; Nemykin, V.N.; Ziegler, C.J. A new highly fluorescent and symmetric pyrrole–BF2 chromophore: BOPHY. J. Am. Chem. Soc. 2014, 136, 5623–5626. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.J.; Jiao, L.J.; Zhang, P.; Feng, Z.Y.; Cheng, C.; Wei, Y.; Mu, X.L.; Hao, E.H. Highly fluorescent BF2 complexes of hydrazine–Schiff base linked bispyrrole. Org. Lett. 2014, 16, 3048–3051. [Google Scholar] [CrossRef] [PubMed]
- Huaulmé, Q.; Mirloup, A.; Retailleau, P.; Ziessel, R. Synthesis of highly functionalized BOPHY chromophores displaying large stokes shifts. Org. Lett. 2015, 17, 2246–2249. [Google Scholar] [CrossRef] [PubMed]
- Rhoda, H.M.; Chanawanno, K.; King, A.J.; Zatsikha, Y.V.; Ziegler, C.J.; Nemykin, V.N. Unusually Strong Long Distance Metal-Metal Coupling in Bis (ferrocene) Containing BOPHY: An Introduction to Organometallic BOPHYs. Chem. Eur. J. 2015, 21, 18043–18046. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tamgho, I.S.; Crandall, L.; Rack, J.; Ziegler, C. Ultrafast dynamics of a new class of highly fluorescent boron difluoride dyes. Phys. Chem. Chem. Phys. 2015, 17, 2349–2351. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, A.R.; Sariki, S.K.; Reddy, R.V.R.; Bisai, A.; Sahu, P.K.; Tomar, R.S.; Sankar, J. Zwitterionic BODIPYs with large stokes shift: Small molecular biomarkers for live cells. Chem. Commun. 2017, 53, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, D.F.; Gao, H.Z.; Zhang, C.; Ni, F.F.; Zhao, W.Q.; Cheng, D.D.; Liu, X.L.; Han, A.X. β-Furan-Fused bis (Difluoroboron)-1,2-bis ((1H-pyrrol-2-yl) methylene) hydrazine Fluorescent Dyes in the Visible Deep-Red Region. J. Org. Chem. 2016, 81, 7439–7447. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Zhou, H.P.; Yin, S.H.; Jiang, H.; Niu, N.; Huang, H.; Shahzad, S.A.; Yu, C. A BOPHY probe for the fluorescence turn-on detection of Cu2+. Sens. Actuators B 2016, 235, 33–38. [Google Scholar] [CrossRef]
- Jiang, X.D.; Su, Y.J.; Yue, S.; Li, C.; Yu, H.F.; Zhang, H.; Sun, C.L.; Xiao, L.J. Synthesis of mono-(p-dimethylamino)styryl-containing BOPHY dye for a turn-on pH sensor. RSC Adv. 2015, 5, 16735–16739. [Google Scholar] [CrossRef]
- Xu, Z.C.; Xiao, Y.; Qian, X.H.; Cui, J.N.; Cui, D.W. Ratiometric and selective fluorescent sensor for CuII based on internal charge transfer (ICT). Org. Lett. 2005, 7, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Qian, X.H.; Cui, J.N. Detecting Hg2+ ions with an ICT fluorescent sensor molecule: Remarkable emission spectra shift and unique selectivity. J. Org. Chem. 2006, 71, 4308–4311. [Google Scholar] [CrossRef] [PubMed]
- Bozdemir, O.A.; Guliyev, R.; Buyukcakir, O.; Selcuk, S.; Kolemen, S.; Gulseren, G.; Nalbantoglu, T.; Boyaci, H.; Akkaya, E.U. Selective manipulation of ICT and PET processes in styryl-bodipy derivatives: Applications in molecular logic and fluorescence sensing of metal ions. J. Am. Chem. Soc. 2010, 132, 8029–8036. [Google Scholar] [CrossRef] [PubMed]
- Srikun, D.; Miller, E.W.; Domaille, D.W.; Chang, C.J. An ICT-based approach to ratiometric fluorescence imaging of hydrogen peroxide produced in living cells. J. Am. Chem. Soc. 2008, 130, 4596–4597. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, V.; Ramamurthy, P.; Thirumalai, D.; Ramakrishnan, V.T. A novel colorimetric and fluorescent chemosensor for anions involving PET and ICT pathways. Org. Lett. 2005, 7, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Q.; Liu, X.L.; Lv, H.T.; Fu, H.; Yang, Y.; Huang, Z.P.; Han, A.X. A phenothiazine–rhodamine ratiometric fluorescent probe for Hg2+ based on FRET and ICT. Tetrahedron Lett. 2015, 56, 4293–4298. [Google Scholar] [CrossRef]








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, D.; Liu, X.; Xie, Y.; Lv, H.; Wang, Z.; Yang, H.; Han, A.; Yang, X.; Zang, L. A Ratiometric Fluorescent Sensor for Cd2+ Based on Internal Charge Transfer. Sensors 2017, 17, 2517. https://doi.org/10.3390/s17112517
Cheng D, Liu X, Xie Y, Lv H, Wang Z, Yang H, Han A, Yang X, Zang L. A Ratiometric Fluorescent Sensor for Cd2+ Based on Internal Charge Transfer. Sensors. 2017; 17(11):2517. https://doi.org/10.3390/s17112517
Chicago/Turabian StyleCheng, Dandan, Xingliang Liu, Yadian Xie, Haitang Lv, Zhaoqian Wang, Hongzhi Yang, Aixia Han, Xiaomei Yang, and Ling Zang. 2017. "A Ratiometric Fluorescent Sensor for Cd2+ Based on Internal Charge Transfer" Sensors 17, no. 11: 2517. https://doi.org/10.3390/s17112517
APA StyleCheng, D., Liu, X., Xie, Y., Lv, H., Wang, Z., Yang, H., Han, A., Yang, X., & Zang, L. (2017). A Ratiometric Fluorescent Sensor for Cd2+ Based on Internal Charge Transfer. Sensors, 17(11), 2517. https://doi.org/10.3390/s17112517