Biosensors Platform Based on Chitosan/AuNPs/Phthalocyanine Composite Films for the Electrochemical Detection of Catechol. The Role of the Surface Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
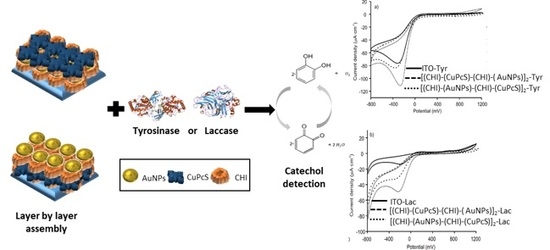
2.2. LbL Film Platforms
- (1)
- Film sequence [(CHI)-(AuNPs)-(CHI)-(CuPcS)]2: CHI solution (5 min) → ultrapure water gently stirred to remove excess of adsorbed CHI (1 min) → AuNPs (5 min)→ ultrapure water gently stirred to remove excess of adsorbed AuNPs → CHI solution (5 min) → Ultrapure water gently stirred to remove excess of adsorbed CHI (1 min) → CuPcS (5 min)→ ultrapure water gently stirred to remove excess of adsorbed CuPcS (1 min).
- (2)
- Film sequence [(CHI)-(CuPcS)-(CHI)-(AuNPs)]2: CHI solution (5 min) → ultrapure water gently stirred to remove excess of adsorbed CHI (1 min) → CuPcS (5 min) → ultrapure water gently stirred to remove excess of adsorbed CuPcS (1 min) → CHI solution (5 min) → ultrapure water gently stirred to remove excess of adsorbed CHI (1 min) → AuNPs (5 min)→ ultrapure water gently stirred to remove excess of adsorbed AuNPs (1 min). In all cases, multilayered LbL films were grown, repeating the “four-step sequence” twice. Films with the sequences [(CHI)-(AuNPs)]2 or [(CHI)-(CuPcS)]2 were also prepared for comparison purposes following the same methodology.
2.3. Preparation of Biosensors LbL-Tyr or LbL-Lac
- Biosensors on film sequence 1: [(CHI)-(AuNPs)-(CHI)-(CuPcS)]2–Tyr; [(CHI)-(AuNPs)-(CHI)-(CuPcS)]2 –Lac.
- Biosensors on film sequence 2: [(CHI)-(CuPcS)-(CHI)-(AuNPs)]2–Tyr; [(CHI)-(CuPcS)-(CHI)-(AuNPs)]2 –Lac.
2.4. Characterization Techniques
3. Results and Discussion
3.1. Preparation and Spectroscopic Characterization of LbL Film Platforms
3.2. Surface Characterization
3.3. Electrochemical Characterization of the LbL-Based Biosensors
3.4. Reproducibility and Repeatability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Soto-Hernandez, M.; Palma Tenango, M.; García-Mateos, M.R. Phenolic Compounds Natural Sources, Importance and Applications; Intechopen: London, UK, 2017. [Google Scholar]
- Lost, R.M.; Crespilho, F.N. Layer-by-layer self-assembly and electrochemistry: Applications in biosensing and bioelectronics. Biosens. Bioelectron. 2012, 31, 1–10. [Google Scholar]
- Oliveira, R.F.; Barros, A.; Ferreira, M. Nanostructured Films: Langmuir–Blodgett (LB) and Layer-by-Layer (LbL) Techniques. J. Nanostructur. 2017, 4, 105–123. [Google Scholar]
- Brett, C.M.A. Perspectives and challenges for self-assembled layer-by-layer biosensor and biomaterial architectures. Curr. Opin. Electrochem. 2018, 12, 21–26. [Google Scholar] [CrossRef]
- Paudyal, S.; Sharma, S.K.; da Silva, R.L.C.G.; Mintz, K.J.; Liyanage, P.Y.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Tyrosinase enzyme Langmuir monolayer: Surface chemistry and spectroscopic study. J. Colloid Interface Sci. 2020, 564, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 16, 8778–8881. [Google Scholar] [CrossRef]
- Karim, M.N.; Lee, J.E.; Lee, H.J. Amperometric detection of catechol using tyrosinase modified electrodes enhanced by the layer-by-layer assembly of gold nanocubes and polyelectrolytes. Biosens. Bioelectron. 2014, 61, 147–151. [Google Scholar] [CrossRef]
- Yang, J.; Li, D.; Fu, J.; Huang, F.; Wei, Q. TiO2-CuCNFs based laccase biosensor for enhanced electrocatalysis in hydroquinone detection. Electroanal. Chem. 2016, 766, 16–23. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; García-Cabezón, C.; García-Hernández, C.; Bramorski, C.; Blanco-Val, Y.; Martín-Pedrosa, F.; Kawai, T.; de Saja, J.A.; Rodríguez-Méndez, M.L. Analysis of organic acids and phenols of interest in the wine industry using Langmuir-Blodgett films based on functionalized nanoparticles. Anal. Chim. Acta 2015, 853, 572–578. [Google Scholar] [CrossRef]
- Taurino, I.; Sanzò, G.; Antiochia, R.; Tortolini, C.; Mazzei, F.; Favero, G.; Micheli, G.; Carrara, S. Recent advances in Third Generation Biosensors based on Au and Pt Nanostructured Electrodes. Trends Anal. Chem. 2016, 79, 151–159. [Google Scholar] [CrossRef]
- Komathi, S.; Gopalan, A.I.; Lee, K.P. Fabrication of a novel layer-by-layer film based glucose biosensor with compact arrangement of multi-components and glucose oxidase. Biosens. Bioelectron. 2009, 24, 3131–3134. [Google Scholar] [CrossRef]
- Andre, R.S.; Shimizu, F.M.; Miyazaki, C.M.; Riul, A., Jr.; Manzani, D.; Ribeiro, S.J.L.; Oliveira, S.N., Jr.; Mattoso, L.H.C.; Correa, D.S. Hybrid layer-by-layer (LbL) films of polyaniline, graphene oxide and zinc oxide to detect ammonia. Sens. Act. B Chem. 2017, 238, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, Q.; Xue, H.; Pang, H. Metal–organic frameworks for electrochemical applications. Coord. Chem. Rev. 2018, 376, 292–318. [Google Scholar] [CrossRef]
- Burger, A.; Kunzmann, A.; Costa, R.D.; Srikantharajah, R.; Peukert, W.; Guldi, D.M.; Hirsch, A. Synergy of Catechol-Functionalized Zinc Oxide Nanorods and Porphyrins in Layer-by-Layer Assemblies. Chem. Eur. J. 2018, 24, 7896–7905. [Google Scholar] [CrossRef] [PubMed]
- Dassie Maximino, M.; Silva Martin, C.; Vieira, F.; Paulovich, P.; Alessio, P. Layer-by-Layer Thin Film of Iron Phthalocyanine as a Simple and Fast Sensor for Polyphenol Determination in Tea Samples. J. Food Sci. 2016, 81, C2344–C2351. [Google Scholar] [CrossRef]
- Gonzalez-Anton, R.; Osipova, M.M.; Garcia-Hernandez, C.; Dubinina, T.V.; Tomilova, G.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Subphthalocyanines as electron mediators in biosensors based on phenol oxidases: Application to the analysis of red wines. Electrochim. Acta 2017, 255, 239–247. [Google Scholar] [CrossRef]
- Fernandes, E.G.R.; Brazaca, L.C.; Rodríguez-Mendez, M.L.; de Saja, J.A.; Zucolotto, V. Immobilization of lutetium bisphthalocyanine in nanostructured biomimetic sensors using the LbL technique for phenol detection, Biosens. Bioelectron. 2011, 26, 4715–4719. [Google Scholar] [CrossRef]
- Delezuka, J.A.M.; Pavinatto, A.; Moraes, M.L.; Shimizu, F.M.; Rodrigues, V.C.; Campana-Filho, S.P.; Ribeiro, S.J.L.; Oliveira, O.N., Jr. Silk fibroin organization induced by chitosan in layer-by-layer films: Application as a matrix in a biosensor. Carbohyd. Polym. 2017, 155, 146–151. [Google Scholar] [CrossRef]
- Barsan, M.M.; David, M.; Florescu, M.; Tugulea, L.; Brett, C.M.A. A new self-assembled layer-by-layer glucose biosensor based on chitosan biopolymer entrapped enzyme with nitrogen doped graphene. Bioelectrochemistry 2014, 99, 46–52. [Google Scholar] [CrossRef]
- Boujakhrout, A.; Jimenez-Falcao, S.; Martinez-Ruiz, P.; Sanchez, A.; Diez, P.; Pingarron, J.M.; Villalonga, R. A Novel reduced graphene oxide-glycol chitosan nanohybrid for the assembly of an amperometric enzyme biosensor for phenols. Analyst 2016, 141, 4162–4169. [Google Scholar] [CrossRef]
- Liu, L.J.; Zhang, F.; Xi, F.N.; Lin, X.F. Highly sensitive biosensor based on bionanomultilayer with water-soluble multiwall carbon nanotubes for determination of phenolics. Biosens. Bioelectron. 2008, 24, 306–312. [Google Scholar] [CrossRef]
- Apetrei, C.; Rodríguez-Méndez, M.L.; de Saja, J.A. Amperometric tyrosinase based biosensor using an electropolymerized phosphate-doped polypyrrole film as an immobilization support. Application for detection of phenolic compounds. Electrochim. Acta 2011, 56, 8919–8925. [Google Scholar] [CrossRef]
- Muthukumar, P.; John, S.A. Synergistic effect of gold nanoparticles and amine functionalized cobalt porphyrin on electrochemical oxidation of hydrazine. New J. Chem. 2014, 38, 3473–3479. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; Furini, L.N.; Constantino, C.J.L.; de Saja, J.A.; Rodriguez-Mendez, M.L. Synergistic electrocatalytic effect of nanostructured mixed films formed by functionalised gold nanoparticles and bisphthalocyanines. Anal. Chim. Acta 2014, 851, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Carmuega, A.I.; Garcia-Hernandez, C.; Ortiz, J.; Garcia-Cabezon, C.; Martin-Pedrosa, F.; Sastre-Santos, A.; Rodriguez-Mendez, M.L. Synergistic effects in electrochemical sensors modified with combinations of sulfur containing phthalocyanines and capped gold nanoparticles. Nanomaterials 2019, 9, 1506–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alencar, W.S.; Crespilho, F.N.; Martins, M.V.A.; Zucolotto, V.; Oliveira, O.N., Jr.; Silva, W.C. Synergistic interaction between gold nanoparticles and nickel phthalocyanine in layer-by-layer (LbL) films: Evidence of constitutional dynamic chemistry (CDC). Phys. Chem. Chem. Phys. 2009, 11, 5086–5091. [Google Scholar] [CrossRef] [PubMed]
- Coros, M.; Pogacean, F.; Magerusan, L.; Rosu, M.C.; Porav, A.S.; Socaci, C.; Bende, A.; Stefan-van Staden, R.I.; Pruneanu, S. Graphene-porphyrin composite synthesis through graphite exfoliation: The electrochemical sensing of catechol, Sens. Actuators B Chem. 2018, 256, 665–673. [Google Scholar] [CrossRef]
- Rajesh, K.; Santhanalakshmi, J. Design and development of graphene intercalated V2O5 nanosheets based electrochemical sensors for effective determination of potentially hazardous 3,5-Dichlorophenol. Mat. Chem. Phys. 2017, 199, 497–507. [Google Scholar] [CrossRef]
- Salvo-Comino, C.; Garcia Hernandez, C.; Garcia-Cabezon, C.; Martin-Pedrosa, F.; Rodriguez-Mendez, M.L. Promoting laccase sensing activity for catechol detection using LbL assemblies as immobilization surfaces. Bioelectrochemistry 2020, 132, 107407. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R.; Kirkland, A.I.; Logan, D.E. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid-Liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- Haiss, W.; Thanh NT, K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV-Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Bertoncello, P.; Peruffo, M. An investigation on the self-aggregation properties of sulfonated copper(II) phthalocyanine (CuTsPc) thin films. Colloids Surf. A 2008, 321, 106–112. [Google Scholar] [CrossRef]
- Frost, S.; Dempsey, M.J.; Whitehead, D.E. The response of citrate functionalised gold and silver nanoparticles to the addition of heavy metal ions. Colloids Surf. A 2017, 518, 15–24. [Google Scholar] [CrossRef]
- Kumar, S.; Barth, A. Following Enzyme Activity with Infrared Spectroscopy. Sensors 2010, 10, 2626–2637. [Google Scholar] [CrossRef] [Green Version]
- Polyanskaya, T.M.; Khaldoyanidi, K.A.; Smolentsev, A.I. Supramolecular architecture of catechol and its 2:1 complex with dimethylsulfoxide. J. Struct. Chem. 2010, 51, 327–334. [Google Scholar] [CrossRef]
- Prehn, R.; Gonzalo-Ruiz, J.; Cortina-Puig, M. Electrochemical Detection of Polyphenolic Compounds in Foods and Beverages. Anal. Chem. 2012, 8, 472–484. [Google Scholar] [CrossRef]
- Evans, S.A.G.; Elliott, J.M.; Andrews, L.M.; Bartlett, P.N.; Doyle, P.N.; Doyle, P.J.; Denuault, G. Detection of Hydrogen Peroxide at MesoporousPlatinum Microelectrodes. Anal. Chem. 2002, 74, 1322–1326. [Google Scholar] [CrossRef]
- Albuquerque, Y.D.T.; Ferreira, L.F. Amperometric biosensing of carbamate and organophosphate pesticidesutilizing screen-printed tyrosinase-modified electrodes. Anal. Chim. Acta 2007, 596, 210–221. [Google Scholar] [CrossRef]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gila, E. Fast and direct amperometric analysis of polyphenols in beers using tyrosinase-modified screen-printed gold nanoparticles biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef]
- Albayati, S.A.R.; Kashanian, S.; Nazari, M.; Rezaei, S. Novel fabrication of a laccase biosensor to detect phenolic compounds using a carboxylated multiwalled carbon nanotubeon the electropolymerized support. Bull. Mat. Sci. 2019, 42, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Medina-Plaza, C.; de Saja, J.A.; Rodriguez-Mendez, M.L. Bioelectronic tongue based on lipidic nanostructured layers containing phenol oxidases and lutetium bisphthalocyanine for the analysis of grapes. Biosens. Bioelectron. 2014, 57, 276–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apetrei, C.; Alessio, P.; Constantino, C.J.L.; de Saja, J.A.; Rodriguez-Mendez, M.L.; Pavinatto, F.J.; Ramos-Fernandes, E.G.; Zucolotto, V.; Oliveira, O.N., Jr. Biomimetic biosensor based on lipidic layers containing tyrosinase and lutetiumbisphthalocyanine for the detection of antioxidants. Biosens. Bioelectron. 2011, 26, 2513–2519. [Google Scholar] [CrossRef] [PubMed]
- Ozsoz, M.; Erdern, A.; Kilinc, E.; Gokgunnec, L. Mushroom-Based Cobalt Phthalocyanine Dispersed Amperometric Biosensor for the Determination of Phenolic Compounds. Electroanalysis 1996, 8, 147–150. [Google Scholar] [CrossRef]
- Polatoglu, I. Electrochemical Sensing Platform Based on TyrosinaseImmobilized Magnetite Chitosan Nanobiocomposite Film and ItsApplication as Catechol Biosensor. J. Electrochem. Soc. 2019, 166, B1620–B1629. [Google Scholar] [CrossRef]
- Bracamonte, M.V.; Linárez-Pérez, O.E.; López-Teijelo, M.; Rivas, G.A.; Ferreyra, N. Quaternized chitosan mediated assembly of gold nanoparticles multilayers. Electrochim. Acta 2014, 146, 178–185. [Google Scholar] [CrossRef]





| Biosensor Description | R 2 | Sensitivity (A M−1) | LOD (µM) | Linear Range (µM) | Ref. | |
|---|---|---|---|---|---|---|
| [(CHI)-( CuPcS)-(CHI)-( AuNPs)]-Tyr | 0.969 | 1.500 | 9.55·10−3 | 2.4–14.9 | 11.04 | This work |
| [(CHI)-(AuNPs)-(CHI)-(CuPcS)]-Tyr | 0.964 | 0.681 | 8.55·10−4 | 2.4–20.0 | 14.41 | This work |
| [(CHI)-( CuPcS)-(CHI)-( AuNPs)]-Lac | 0.991 | 0.384 | 5.89·10−2 | 2.4–16.6 | 8.76 | This work |
| [(CHI)-(AuNPs)-(CHI)-(CuPcS)]-Lac | 0.996 | 0.188 | 1.84·10−2 | 2.4–20.0 | 7.45 | This work |
| Tyr-AuNP-SPCE | -- | 0.08 | 0.2 | 0.8–120.0 | -- | [7] |
| [CHI (+) +IL (+) |CuPc S(-)] 2 |Lac | 0.981 | 0.230 | 9.98·10−3 | 2.4–14.9 | 3.16 | [29] |
| Tyr-AuNPs-SPCE | 0.993 | 0.55 | 1.2 | 2.5–20.0 | -- | [39] |
| CoPc-CGCE-Tyr | 0.991 | 0.160 | 4.50·10−1 | 5.0–1000.0 | 1600.00 | [40] |
| Lac/MWCNT/AuNPs-SDBS-PEDOT/GCE | 0.960 | 0.012 | 1.10·10−1 | 0.1–0.5 | -- | [41] |
| Lac/AA/LuPc 2 | 0.992 | -- | 4.88·10−1 | 4.0–150.0 | -- | [42] |
| Tyr/AA/LuPc 2 | 0.997 | -- | 5.18·10−1 | 4.0–150.0 | -- | [42] |
| Tyr/AA/LuPc 2 | -- | -- | 1.71 | 1.9–27.5 | 63.72 | [43] |
| CoPc-CPEs-Tyr | -- | 0.002 | 7.5 | 30.0–320.0 | 120.00 | [44] |
| Tyr-magnetite-CHI-GCE | 0.996 | 0.057 | 2.62·10−1 | 1.0–30.0 | 22.5 | [45] |
| MWCNT/Lac/CHI-CPE | 0.999 | 0.279 | 3.34·10−2 | 0.1–165.0 | -- | [46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvo-Comino, C.; González-Gil, A.; Rodriguez-Valentin, J.; Garcia-Hernandez, C.; Martin-Pedrosa, F.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Biosensors Platform Based on Chitosan/AuNPs/Phthalocyanine Composite Films for the Electrochemical Detection of Catechol. The Role of the Surface Structure. Sensors 2020, 20, 2152. https://doi.org/10.3390/s20072152
Salvo-Comino C, González-Gil A, Rodriguez-Valentin J, Garcia-Hernandez C, Martin-Pedrosa F, Garcia-Cabezon C, Rodriguez-Mendez ML. Biosensors Platform Based on Chitosan/AuNPs/Phthalocyanine Composite Films for the Electrochemical Detection of Catechol. The Role of the Surface Structure. Sensors. 2020; 20(7):2152. https://doi.org/10.3390/s20072152
Chicago/Turabian StyleSalvo-Comino, Coral, Alfonso González-Gil, Javier Rodriguez-Valentin, Celia Garcia-Hernandez, Fernando Martin-Pedrosa, Cristina Garcia-Cabezon, and Maria Luz Rodriguez-Mendez. 2020. "Biosensors Platform Based on Chitosan/AuNPs/Phthalocyanine Composite Films for the Electrochemical Detection of Catechol. The Role of the Surface Structure" Sensors 20, no. 7: 2152. https://doi.org/10.3390/s20072152
APA StyleSalvo-Comino, C., González-Gil, A., Rodriguez-Valentin, J., Garcia-Hernandez, C., Martin-Pedrosa, F., Garcia-Cabezon, C., & Rodriguez-Mendez, M. L. (2020). Biosensors Platform Based on Chitosan/AuNPs/Phthalocyanine Composite Films for the Electrochemical Detection of Catechol. The Role of the Surface Structure. Sensors, 20(7), 2152. https://doi.org/10.3390/s20072152