A Simple, Reliable, and Inexpensive Solution for Contact Color Measurement in Small Plant Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
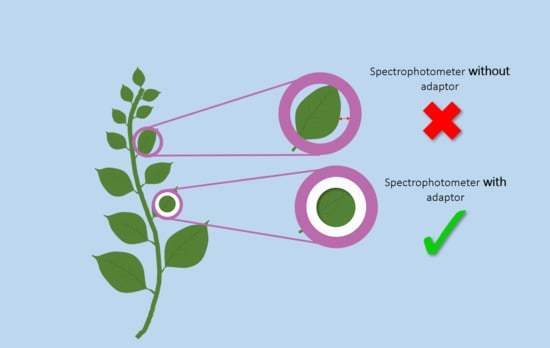
2.2. Color Measurements with and Without the Adaptors
2.3. Colorimetric Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Jasione montana | Trachelium caeruleum | Campanula rotundifolia | Campanula isophylla | Hesperocodon hederacerus | Jasione laevis | ||||||||
| MAV | 50% black | ΔL*: 3.4 Δa*: 4.5 Δb*: 7.6 | ΔE*ab: 9.5 | ΔL*: 2.2 Δa*: 3.7 Δb*: 6.1 | ΔE*ab: 7.4 | ΔL*: 0.9 Δa*: 2.6 Δb*: 3.5 | ΔE*ab: 4.5 | ΔL*: 0.6 Δa*: 2.5 Δb*: 2.8 | ΔE*ab: 3.8 | ΔL*: 2.4 Δa*: 2.7 Δb*: 4.3 | ΔE*ab: 5.6 | ΔL*: 1.9 Δa*: 2.9 Δb*: 3.9 | ΔE*ab: 5.3 |
| 50% white | ΔL*: 17.3 Δa*: 9.7 Δb*: 15.2 | ΔE*ab: 25.0 | ΔL*: 20.6 Δa*: 9.4 Δb*: 13.0 | ΔE*ab: 26.1 | ΔL*: 20.4 Δa*: 8.4 Δb*: 9.6 | ΔE*ab: 24.1 | ΔL*: 22.3 Δa*: 7.5 Δb*: 6.9 | ΔE*ab: 24.5 | ΔL*: 18.5 Δa*: 8.2 Δb*: 10.4 | ΔE*ab: 22.8 | ΔL*: 22.0 Δa*: 6.9 Δb*: 6.5 | ΔE*ab: 23.9 | |
| 70% black | ΔL*: 11.8 Δa*: 9.6 Δb*: 16.1 | ΔE*ab: 22.1 | ΔL*: 8.7 Δa*: 9.4 Δb*: 14.2 | ΔE*ab: 19.1 | ΔL*: 7.4 Δa*: 7.7 Δb*: 10.5 | ΔE*ab: 15.0 | ΔL*: 5.9 Δa*: 7.0 Δb*: 8.3 | ΔE*ab: 12.4 | ΔL*: 9.8 Δa*: 7.9 Δb*: 12.0 | ΔE*ab: 17.4 | ΔL*: 6.5 Δa*: 6.6 Δb*: 8.1 | ΔE*ab: 12.3 | |
| 70% white | ΔL*: 37.0 Δa*: 14.8 Δb*: 23.8 | ΔE*ab: 46.4 | ΔL*: 41.5 Δa*: 13.9 Δb*: 20.5 | ΔE*ab: 48.3 | ΔL*: 42.3 Δa*: 12.0 Δb*: 15.8 | ΔE*ab: 46.8 | ΔL*: 45.1 Δa*: 10.5 Δb*: 12.1 | ΔE*ab: 47.8 | ΔL*: 39.6 Δa*: 12.4 Δb*: 17.8 | ΔE*ab: 45.1 | ΔL*: 44.9 Δa*: 9.2 Δb*: 10.8 | ΔE*ab: 47.1 | |
| SAV | 50% black | ΔL*: 1.7 Δa*: 0.6 Δb*: 1.8 | ΔE*ab: 2.5 | ΔL*: 1.2 Δa*: 0.8 Δb*: 1.9 | ΔE*ab 2.4 | ΔL*: 1.2 Δa*: 0.7 Δb*: 1.4 | ΔE*ab: 2.0 | ΔL*: 0.1 Δa*: 0.5 Δb*: 0.7 | ΔE*ab: 0.8 | ΔL*: 0.0 Δa*: 0.2 Δb*: 0.6 | ΔE*ab: 0.7 | ΔL*: 0.1 Δa*: 0.7 Δb*: 1.0 | ΔE*ab: 1.2 |
| 50% white | ΔL*: 0.9 Δa*: 0.9 Δb*: 1.7 | ΔE*ab: 2.1 | ΔL*: 0.7 Δa*: 1.2 Δb*: 2.3 | ΔE*ab: 2.7 | ΔL*: 0.6 Δa*: 1.0 Δb*: 1.3 | ΔE*ab: 1.8 | ΔL*: 0.6 Δa*: 0.7 Δb*: 0.6 | ΔE*ab: 1.1 | ΔL*: 0.9 Δa*: 0.3 Δb*: 0.5 | ΔE*ab: 1.1 | ΔL*: 0.6 Δa*: 0.9 Δb*: 1.0 | ΔE*ab: 1.4 | |
| 70% black | ΔL*: 9.4 Δa*: 9.3 Δb*: 16.5 | ΔE*ab: 21.1 | ΔL*: 6.0 Δa*: 10.0 Δb*: 15.8 | ΔE*ab: 19.6 | ΔL*: 6.9 Δa*: 8.3 Δb*: 11.6 | ΔE*ab: 15.9 | ΔL*: 3.9 Δa*: 7.3 Δb*: 8.3 | ΔE*ab: 11.7 | ΔL*: 7.3 Δa*: 8.0 Δb*: 12.0 | ΔE*ab: 16.1 | ΔL*: 4.2 Δa*: 6.6 Δb*: 7.7 | ΔE*ab: 11.0 | |
| 70% white | ΔL*: 24.6 Δa*: 11.7 Δb*: 21.0 | ΔE*ab: 34.4 | ΔL*: 27.6 Δa*: 12.1 Δb*: 19.8 | ΔE*ab: 36.1 | ΔL*: 29.5 Δa*: 10.8 Δb*: 14.1 | ΔE*ab: 34.4 | ΔL*: 33.1 Δa*: 9.4 Δb*: 10.1 | ΔE*ab: 35.9 | ΔL*: 29.2 Δa*: 10.7 Δb*: 15.1 | ΔE*ab: 34.6 | ΔL*: 32.7 Δa*: 8.2 Δb*: 9.0 | ΔE*ab: 34.9 | |
| Campanula adsurgens * | Campanula latifolia * | Campanula mollis * | Campanula glomerata * | Campanula trachelium * | Campanula vesicular * | ||||||||
| MAV | 50% black | ΔL*: 6.2 Δa*: 0.4 Δb*: 4.1 | ΔE*ab: 7.4 | ΔL*: 6.6 Δa*: 0.8 Δb*: 5.4 | ΔE*ab: 8.5 | ΔL*: 0.8 Δa*: 0.4 Δb*: 4.0 | ΔE*ab: 4.1 | ΔL*: 3.3 Δa*: 2.0 Δb*: 5.2 | ΔE*ab: 6.5 | ΔL*: 5.3 Δa*: 1.6 Δb*: 7.9 | ΔE*ab: 9.7 | ΔL*: 3.0 Δa*: 0.6 Δb*: 4.5 | ΔE*ab: 5.4 |
| 50% white | ΔL*: 11.3 Δa*: 0.8 Δb*: 9.5 | ΔE*ab: 14.8 | ΔL*: 15.4 Δa*: 0.9 Δb*: 10.3 | ΔE*ab: 18.6 | ΔL*: 14.8 Δa*: 0.4 Δb*: 5.8 | ΔE*ab: 15.9 | ΔL*: 15.0 Δa*: 5.4 Δb*: 12.7 | ΔE*ab: 20.4 | ΔL*: 13.3 Δa*: 2.1 Δb*: 10.5 | ΔE*ab: 17.1 | ΔL*: 15.8 Δa*: 0.5 Δb*: 10.7 | ΔE*ab: 19.1 | |
| 70% black | ΔL*: 16.5 Δa*: 0.4 Δb*: 11.1 | ΔE*ab: 19.9 | ΔL*: 13.4 Δa*: 1.5 Δb*: 12.0 | ΔE*ab: 18.1 | ΔL*: 15.5 Δa*: 0.2 Δb*: 8.0 | ΔE*ab: 17.4 | ΔL*: 14.1 Δa*: 5.3 Δb*: 14.6 | ΔE*ab: 21.0 | ΔL*: 16.6 Δa*: 2.9 Δb*: 12.7 | ΔE*ab: 21.1 | ΔL*: 14.3 Δa*: 0.9 Δb*: 13.7 | ΔE*ab: 19.8 | |
| 70% white | ΔL*: 29.2 Δa*: 0.9 Δb*: 18.3 | ΔE*ab: 34.5 | ΔL*: 35.8 Δa*: 1.3 Δb*: 18.1 | ΔE*ab: 40.1 | ΔL*: 32.8 Δa*: 0.9 Δb*: 12.8 | ΔE*ab: 35.2 | ΔL*: 33.9 Δa*: 8.8 Δb*: 22.7 | ΔE*ab: 41.8 | ΔL*: 32.2 Δa*: 3.3 Δb*: 19.0 | ΔE*ab: 37.5 | ΔL*: 33.2 Δa*: 0.2 Δb*: 20.6 | ΔE*ab: 39.0 | |
| SAV | 50% black | ΔL*: 0.0 Δa*: 0.1 Δb*: 2.1 | ΔE*ab: 2.1 | ΔL*: 2.3 Δa*: 0.2 Δb*: 1.8 | ΔE*ab: 2.9 | ΔL*: 0.8 Δa*: 0.2 Δb*: 1.2 | ΔE*ab: 1.5 | ΔL*: 1.6 Δa*: 0.6 Δb*: 1.8 | ΔE*ab: 2.4 | ΔL*: 2.5 Δa*: 0.5 Δb*: 1.3 | ΔE*ab: 2.9 | ΔL*: 1.7 Δa*: 0.2 Δb*: 1.2 | ΔE*ab: 2.1 |
| 50% white | ΔL*: 0.2 Δa*: 0.6 Δb*: 0.9 | ΔE*ab: 1.1 | ΔL*: 2.0 Δa*: 0.0 Δb*: 1.5 | ΔE*ab: 2.4 | ΔL*: 2.3 Δa*: 0.2 Δb*: 1.6 | ΔE*ab: 2.8 | ΔL*: 0.1 Δa*: 0.9 Δb*: 2.2 | ΔE*ab: 2.4 | ΔL*: 1.2 Δa*: 0.3 Δb*: 1.6 | ΔE*ab: 2.0 | ΔL*: 0.3 Δa*: 0.2 Δb*: 0.6 | ΔE*ab: 0.7 | |
| 70% black | ΔL*: 14.9 Δa*: 0.1 Δb*: 11.6 | ΔE*ab: 18.9 | ΔL*: 12.8 Δa*: 1.5 Δb*: 11.9 | ΔE*ab: 17.6 | ΔL*: 16.3 Δa*: 0.2 Δb*: 5.8 | ΔE*ab: 17.3 | ΔL*: 10.9 Δa*: 4.6 Δb*: 14.1 | ΔE*ab: 18.4 | ΔL*: 13.9 Δa*: 3.0 Δb*: 13.4 | ΔE*ab: 19.5 | ΔL*: 13.7 Δa*: 0.6 Δb*: 13.0 | ΔE*ab: 18.9 | |
| 70% white | ΔL*: 25.1 Δa*: 0.6 Δb*: 17.5 | ΔE*ab: 30.6 | ΔL*: 30.4 Δa*: 1.6 Δb*: 17.5 | ΔE*ab: 35.1 | ΔL*: 24.2 Δa*: 0.4 Δb*: 10.6 | ΔE*ab: 26.4 | ΔL*: 29.6 Δa*: 7.2 Δb*: 21.4 | ΔE*ab: 37.2 | ΔL*: 28.0 Δa*: 3.5 Δb*: 19.1 | ΔE*ab: 34.0 | ΔL*: 27.4 Δa*: 0.2 Δb*: 19.1 | ΔE*ab: 33.4 | |
| Green | Foliage | Yellow Green | Bluish Green | ||||||||||
| MAV | 50% black | ΔL*: 4.6 Δa*: 7.1 Δb*: 6.5 | ΔE*ab: 10.7 | ΔL*: 2.5 Δa*: 3.3 Δb*: 5.2 | ΔE*ab: 6.6 | ΔL*: 7.2 Δa*: 3.6 Δb*: 9.8 | ΔE*ab: 12.7 | ΔL*: 6.6 Δa*: 4.4 Δb*: 0.6 | ΔE*ab: 7.9 | ||||
| 50% white | ΔL*: 11.3 Δa*: 19.3 Δb*: 15.4 | ΔE*ab: 27.1 | ΔL*: 17.1 Δa*: 8.9 Δb*: 11.3 | ΔE*ab: 22.3 | ΔL*: 4.9 Δa*: 9.9 Δb*: 23.9 | ΔE*ab: 26.3 | ΔL*: 5.7 Δa*: 11.8 Δb*: 0.5 | ΔE*ab: 13.1 | |||||
| 70% black | ΔL*: 17.9 Δa*: 22.3 Δb*: 19.4 | ΔE*ab: 34.5 | ΔL*: 11.3 Δa*: 9.6 Δb*: 14.1 | ΔE*ab: 20.4 | ΔL*: 26.1 Δa*: 13.0 Δb*: 30.7 | ΔE*ab: 42.4 | ΔL*: 25.4 Δa*: 15.4 Δb*: 1.1 | ΔE*ab: 29.7 | |||||
| 70% white | ΔL*: 27.7 Δa*: 36.0 Δb*: 29.7 | ΔE*ab: 54.2 | ΔL*: 38.3 Δa*: 14.5 Δb*: 20.0 | ΔE*ab: 45.6 | ΔL*: 13.5 Δa*: 23.8 Δb*: 50.2 | ΔE*ab: 57.2 | ΔL*: 15.3 Δa*: 27.4 Δb*: 1.2 | ΔE*ab: 31.4 | |||||
| SAV | 50% black | ΔL*: 1.6 Δa*: 1.6 Δb*: 1.6 | ΔE*ab: 2.8 | ΔL*: 1.1 Δa*: 0.8 Δb*: 1.3 | ΔE*ab: 1.9 | ΔL*: 2.0 Δa*: 0.7 Δb*: 1.9 | ΔE*ab: 2.9 | ΔL*: 2.3 Δa*: 0.9 Δb*: 0.0 | ΔE*ab: 2.5 | ||||
| 50% white | ΔL*: 0.0 Δa*: 1.0 Δb*: 0.9 | ΔE*ab: 1.3 | ΔL*: 0.3 Δa*: 0.8 Δb*: 1.2 | ΔE*ab: 1.4 | ΔL*: 1.3 Δa*: 0.6 Δb*: 2.2 | ΔE*ab: 2.6 | ΔL*: 0.3 Δa*: 0.3 Δb*: 0.1 | ΔE*ab: 0.5 | |||||
| 70% black | ΔL*: 12.8 Δa*: 20.3 Δb*: 17.4 | ΔE*ab: 29.7 | ΔL*: 6.4 Δa*: 8.7 Δb*: 12.5 | ΔE*ab: 16.5 | ΔL*: 20.3 Δa*: 11.8 Δb*: 28.1 | ΔE*ab: 36.6 | ΔL*: 19.6 Δa*: 13.7 Δb*: 0.2 | ΔE*ab: 23.9 | |||||
| 70% white | ΔL*: 21.1 Δa*: 31.3 Δb*: 28.4 | ΔE*ab: 47.2 | ΔL*: 29.9 Δa*: 13.2 Δb*: 20.7 | ΔE*ab: 38.7 | ΔL*: 9.3 Δa*: 20.0 Δb*: 45.8 | ΔE*ab: 50.9 | ΔL*: 11.3 Δa*: 22.4 Δb*: 2.0 | ΔE*ab: 25.2 | |||||
References
- Rahaman, M.; Chen, D.; Gillani, Z.; Klukas, C.; Chen, M. Advanced phenotyping and phenotype data analysis for the study of plant growth and development. Front. Plant Sci. 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Neumann, K.; Friedel, S.; Kilian, B.; Chen, M.; Altmann, T.; Klukas, C. Dissecting the phenotypic components of crop plant growth and drought responses based on high-throughput image analysis. Plant Cell 2014, 26, 4636–4655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kevan, P.G. Floral colors through the insect eye: What they are and what they mean. In Handbook of Experimental Pollination Biology; Jones, C.E., Little, R.J., Eds.; Van Nostrand Reinhold Company: New York, NY, USA, 1983; pp. 3–30. Available online: https://www.researchgate.net/publication/262221981_FLORAL_COLORS_THROUGH_THE_INSECT_EYE_WHAT_THEY_ARE_AND_WHAT_THEY_MEAN (accessed on 20 April 2020).
- Archetti, M. The Origin of Autumn Colours by Coevolution. J. Theor. Boil. 2000, 205, 625–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, K. Colors of young and old spring leaves as a potential signal for ant-tended hemipterans. Plant Signal. Behav. 2008, 3, 984–985. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-P.; Shih, Y.-T.; Lin, B.-R.; Chiu, C.-F.; Lin, C.-C. Inhibitory Effect and Mechanisms of an Anthocyanins- and Anthocyanidins-Rich Extract from Purple-Shoot Tea on Colorectal Carcinoma Cell Proliferation. J. Agric. Food Chem. 2012, 60, 3686–3692. [Google Scholar] [CrossRef]
- Rashid, K.; Wachira, F.; Nyabuga, J.N.; Wanyonyi, B.; Murilla, G.; Isaac, A.O. Kenyan purple tea anthocyanins ability to cross the blood brain barrier and reinforce brain antioxidant capacity in mice. Nutr. Neurosci. 2013, 17, 178–185. [Google Scholar] [CrossRef]
- Borghesi, E.; González-Miret, M.L.; Escudero, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of Salinity Stress on Carotenoids, Anthocyanins, and Color of Diverse Tomato Genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682. [Google Scholar] [CrossRef]
- Abdelaziz, M.; Muñoz-Pajares, A.J.; Lorite, J.; Herrador, M.B.; Perfectti, F.; Gómez, J.M. Phylogenetic relationships of Erysimum (Brassicaceae) from the Baetic Mountains (SE Iberian Peninsula). Anales del Jardín Botánico de Madrid 2014, 71, e005. [Google Scholar] [CrossRef] [Green Version]
- Valenta, K.; Kalbitzer, U.; Razafimandimby, D.; Omeja, P.; Ayasse, M.; Chapman, C.A.; Nevo, O. The evolution of fruit colour: Phylogeny, abiotic factors and the role of mutualists. Sci. Rep. 2018, 8, 14302. [Google Scholar] [CrossRef] [Green Version]
- Ougham, H.; Thomas, H.; Archetti, M. The adaptive value of leaf colour. New Phytol. 2008, 179, 9–13. [Google Scholar] [CrossRef]
- Yuan, Y.; Sagawa, J.M.; Young, R.C.; Christensen, B.; Bradshaw, H.D. Genetic Dissection of a Major Anthocyanin QTL Contributing to Pollinator-Mediated Reproductive Isolation Between Sister Species of Mimulus. Genetics 2013, 194, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbedo, J.G. Digital image processing techniques for detecting, quantifying and classifying plant diseases. SpringerPlus 2013, 2, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunaga, T.M.; Ogawa, D.; Taguchi-Shiobara, F.; Ishimoto, M.; Matsunaga, S.; Habu, Y. Direct quantitative evaluation of disease symptoms on living plant leaves growing under natural light. Breed. Sci. 2017, 67, 316–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, J.; Abramov, I. Color vision in the peripheral retina. Hue and saturation. J. Opt. Soc. Am. 1977, 6, 202–207. [Google Scholar] [CrossRef]
- Nerger, J.L.; Volbrecht, V.J.; Ayde, C.J. Unique hue judgments as a function of test size in the fovea and at 20-deg temporal eccentricity. J. Opt. Soc. Am. A 1995, 12, 1225. [Google Scholar] [CrossRef]
- CIE. Publication 15.2: Colorimetry, 2nd ed.; Central Bureau: Vienna, Australia, 1986. [Google Scholar] [CrossRef]
- Fairchild, M.D. Color Appearance Models; Addison-Wesley: Reading, MA, USA, 1998. [Google Scholar] [CrossRef]
- Hunt, R.W.G.; Pointer, M.R. Measuring Colour, 4th ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; p. 468. [Google Scholar]
- Lu, F.; Bu, Z.; Lu, S. Estimating Chlorophyll Content of Leafy Green Vegetables from Adaxial and Abaxial Reflectance. Sensors 2019, 19, 4059. [Google Scholar] [CrossRef] [Green Version]
- Prieto, B.; Sanmartín, P.; Silva, B.; Martínez-Verdú, F. Measuring the color of granite rocks: A proposed procedure. Color Res. Appl. 2010, 35, 368–375. [Google Scholar] [CrossRef]
- Bokhari, M.H.; Sales, F. JASIONE (CAMPANULACEAE) ANATOMY IN THE IBERIAN PENINSULA AND ITS TAXONOMIC SIGNIFICANCE. Edinb. J. Bot. 2001, 58, 405–422. [Google Scholar] [CrossRef]
- Nagy, L.; Grabherr, G. The Biology of Alpine Habitats; University of Oxford Press: Oxford, UK, 2009. [Google Scholar]
- Sales, F.; Hedge, I.C.; Jasione, L. Flora iberica XIV. Myoporaceae-Campanulaceae; Paiva, J., Sales, F., Hedge, I.C., Aedo, C., Aldasoro, J.J., Castroviejo, S., Herrero, A., Velayos, M., Eds.; CSIC: Madrid, Spain, 2001; Available online: https://www.editorial.csic.es/publicaciones/libros/4650/978-84-00-07953-6/flora-iberica-vol-xiv-myoporaceae-campanulaceae.html (accessed on 20 April 2020).
- Pérez-Espona, S.; Sales, F.; Hedge, I.; Möller, M. PHYLOGENY AND SPECIES RELATIONSHIPS IN JASIONE (CAMPANULACEAE) WITH EMPHASIS ON THE ‘MONTANA-COMPLEX’. Edinb. J. Bot. 2005, 62, 29–51. [Google Scholar] [CrossRef]
- Byers, A. Contemporary Human Impacts on Alpine Ecosystems in the Sagarmatha (Mt. Everest) National Park, Khumbu, Nepal. Ann. Assoc. Am. Geogr. 2005, 95, 112–140. [Google Scholar] [CrossRef]
- Rixen, C.; Wipf, S.; Frei, E.R.; Stöckli, V. Faster, higher, more? Past, present and future dynamics of alpine and arctic flora under climate change. Alp. Bot. 2014, 124, 77–79. [Google Scholar] [CrossRef] [Green Version]
- GLORIA Coordination. Available online: https://gloria.ac.at/network/gloria_coordination (accessed on 18 March 2020).
- Sanmartín, P.; Devesa-Rey, R.; Prieto, B.; Barral, M.T. Nondestructive assessment of phytopigments in riverbed sediments by the use of instrumental color measurements. J. Soils Sediments 2011, 11, 841–851. [Google Scholar] [CrossRef]
- Sanmartín, P.; Villa, F.; Silva, B.; Cappitelli, F.; Prieto, B. Color measurements as a reliable method for estimating chlorophyll degradation to phaeopigments. Biogeochemistry 2010, 22, 763–771. [Google Scholar] [CrossRef]
- McCamy, C.S.; Marcus, H.; Davidson, J.G. A color-rendition chart. J. Appl. Photogr. Eng. 1976, 2, 95–99. Available online: https://publiclab.org/system/images/photos/000/004/361/original/mccamy1976.pdf (accessed on 20 April 2020).
- Sanmartín, P.; Chorro, E.; Vázquez-Nion, D.; Martínez-Verdú, F.; Prieto, B. Conversion of a digital camera into a non-contact colorimeter for use in stone cultural heritage: The application case to Spanish granites. Measurement 2014, 56, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Vrhel, M.J.; Gershon, R.; Iwan, L.S. Measurement and Analysis of Object Reflectance Spectra. Color Res. Appl. 1994, 19, 4–9. [Google Scholar] [CrossRef]
- Catrysse, P.B.; Wandell, B.A.; El Gamal, A. Comparative analysis of color architectures for image sensors. Electron. Imaging 99 1999, 3650, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Martínez, J.A.; Melgosa, M.; Pérez, M.; Hita, E.; Negueruela, A.I. Visual and instrumental color evaluation in red wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
- Sanmartín, P.; Silva, B.; Prieto, B. Effect of Surface Finish on Roughness, Color, and Gloss of Ornamental Granites. J. Mater. Civ. Eng. 2011, 23, 1239–1248. [Google Scholar] [CrossRef]
- Sanmartín, P.; Vázquez-Nion, D.; Silva, B.; Prieto, B. Spectrophotometric color measurement for early detection and monitoring of greening on granite buildings. Biofouling 2012, 28, 329–338. [Google Scholar] [CrossRef]
- Molada Tebar, A.; Lerma García, J.L.; Marqués Mateu, Á. Camera characterization for improving color archaeological documentation. Color Res. Appl. 2018, 43, 47–57. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 1st ed.; Springer Science & Business Media: Berlin, Germany, 2009; p. 224. [Google Scholar]
- Korkmaz, S.; Goksuluk, D.; Zararsiz, G. MVN: An R Package for Assessing Multivariate Normality. R J. 2014, 6, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer Science and Business Media LLC: New York, NY, USA, 2002; Available online: http://www.stats.ox.ac.uk/pub/MASS4/ (accessed on 20 April 2020).
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. NbClust: An R Package for Determining the Relevant Number of Clusters in a Data Set. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011; ISBN 3-900051-07-0. Available online: http://www.R-project.org/ (accessed on 19 August 2011).
- Suzuki, R.; Shimodaira, H. Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 2006, 22, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Hang, J.; Zhang, D.; Chen, P.; Zhang, J.; Wang, B. Classification of Plant Leaf Diseases Based on Improved Convolutional Neural Network. Sensors 2019, 19, 4161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chianucci, F.; Ferrara, C.; Pollastrini, M.; Corona, P. Development of digital photographic approaches to assess leaf traits in broadleaf tree species. Ecol. Indic. 2019, 106, 105547. [Google Scholar] [CrossRef]
- Kytridis, V.-P.; Karageorgou, P.; Levizou, E.; Manetas, Y. Intra-species variation in transient accumulation of leaf anthocyanins in Cistus creticus during winter: Evidence that anthocyanins may compensate for an inherent photosynthetic and photoprotective inferiority of the red-leaf phenotype. J. Plant Physiol. 2008, 165, 952–959. [Google Scholar] [CrossRef]
- Nikiforou, C.; Nikolopoulos, D.; Manetas, Y. The winter-red-leaf syndrome in Pistacia lentiscus: Evidence that the anthocyanic phenotype suffers from nitrogen deficiency, low carboxylation efficiency and high risk of photoinhibition. J. Plant Physiol. 2011, 168, 2184–2187. [Google Scholar] [CrossRef]
- Cordlandwehr, V.; Meredith, R.L.; Ozinga, W.A.; Bekker, R.M.; Groenendael, J.M.; Bakker, J.P. Do plant traits retrieved from a database accurately predict on-site measurements? J. Ecol. 2013, 101, 662–670. [Google Scholar] [CrossRef]
- Carranza-Rojas, J.; Goëau, H.; Bonnet, P.; Mata-Montero, E.; Joly, A. Going deeper in the automated identification of Herbarium specimens. BMC Evol. Boil. 2017, 17, 181. [Google Scholar] [CrossRef] [Green Version]
- Bedford, D.J. Vascular plants. In Care and Conservation of Natural History Collections; Carter, D., Walker, A., Eds.; Butterwoth Heinemann: Oxford, UK, 1999; pp. 61–80. Available online: https://www.natsca.org/care-and-conservation (accessed on 20 April 2020).
- Parnell, J. Variation in Jasione montana L. (Campanulaceae) and related species in Europe and North Africa. Watsonia 1987, 16, 249–267. Available online: https://www.watsonia.org.uk/html/watsonia_16.html#p249.pdf (accessed on 20 April 2020).
- Serrano, M.; Ortiz, J.P.; Parra, M.B. Presencia y estado de conservación de Jasione corymbosa Poir. ex Schult. (Campanulaceae) en la Península Ibérica. Acta Bot. Malacit. 2019, 34, 284–287. [Google Scholar] [CrossRef]







| Herbarium Code 1. | Species | Location 2 | Collector | Year |
|---|---|---|---|---|
| SANT 210 | Campanula latifolia L. | ES. Peña Oroel, Jaca | Bellot, Vieitez | 1947 |
| SANT 232 | Campanula trachelium L. | ES. Province of Barcelona | Marcos | 1944 |
| SANT 21287 | Campanula adsurgens Levier & Leresche | ES. Triacastela, Lugo | J. Amigo, J. Giménez de Azcárate | 1990 |
| SANT 29493 | Campanula versicolor Andrews | IT. Santa Cesarea, Lecce | J. Izco | 1994 |
| SANT 51406 | Campanula glomerata L. | ES. Penedos de Oulego, Ourense | J. Amigo | 2004 |
| SANT 62512 | Campanula mollis L. | ES. Sierra de Grazalema, Cádiz | J. Izco | 1976 |
| J. montana | T. caeruleum | C. rotundifolia | C. isophylla | H. hederaceus | J. laevis | ||
| MAV | 50% black | 9.5 | 7.4 | 4.5 | 3.8 | 5.6 | 5.3 |
| 50% white | 25.0 | 26.1 | 24.1 | 24.5 | 22.8 | 23.9 | |
| 70% black | 22.1 | 19.1 | 15.0 | 12.4 | 17.4 | 12.3 | |
| 70% white | 46.4 | 48.3 | 46.8 | 47.8 | 45.1 | 47.1 | |
| SAV | 50% black | 2.5 | 2.4 | 2.0 | 0.8 | 0.7 | 1.2 |
| 50% white | 2.1 | 2.7 | 1.8 | 1.1 | 1.1 | 1.4 | |
| 70% black | 21.1 | 19.6 | 15.9 | 11.7 | 16.1 | 11.0 | |
| 70% white | 34.4 | 36.1 | 34.4 | 35.9 | 34.6 | 34.9 | |
| C. adsurgens * | C. latifolia * | C. mollis * | C. glomerate * | C. trachelium * | C. vesicular * | ||
| MAV | 50% black | 7.4 | 8.5 | 4.1 | 6.5 | 9.7 | 5.4 |
| 50% white | 14.8 | 18.6 | 15.9 | 20.4 | 17.1 | 19.1 | |
| 70% black | 19.9 | 18.1 | 17.4 | 21.0 | 21.1 | 19.8 | |
| 70% white | 34.5 | 40.1 | 35.2 | 41.8 | 37.5 | 39.0 | |
| SAV | 50% black | 2.1 | 2.9 | 1.5 | 2.4 | 2.9 | 2.1 |
| 50% white | 1.1 | 2.4 | 2.8 | 2.4 | 2.0 | 0.7 | |
| 70% black | 18.9 | 17.6 | 17.3 | 18.4 | 19.5 | 18.9 | |
| 70% white | 30.6 | 35.1 | 26.4 | 37.2 | 34.0 | 33.4 | |
| Green | Foliage | Yellow Green | Bluish Green | ||||
| MAV | 50% black | 10.7 | 6.6 | 12.7 | 7.9 | ||
| 50% white | 27.1 | 22.3 | 26.3 | 13.1 | |||
| 70% black | 34.5 | 20.4 | 42.4 | 29.7 | |||
| 70% white | 54.2 | 45.6 | 57.2 | 31.4 | |||
| SAV | 50% black | 2.8 | 1.9 | 2.9 | 2.5 | ||
| 50% white | 1.3 | 1.4 | 2.6 | 0.5 | |||
| 70% black | 29.7 | 16.5 | 36.6 | 23.9 | |||
| 70% white | 47.2 | 38.7 | 50.9 | 25.2 | |||
| Fresh Material | Herbarium Material | ||||||
|---|---|---|---|---|---|---|---|
| AU | k | AU | k | ||||
| p-Value | p-Value | ||||||
| Jasione laevis | A = {100% SAV, 100% SAV50W, 100% SAV50B, 100% SAV70W} | 97 | 2 | Campanula adsurgens | A = {100% SAV, 100% SAV50W, 92% SAV50B} | 99 | 3 |
| B = {100% SAV70B} | 97 | B = {100% SAV70W, 8% SAV70B} | 97 | ||||
| C = {10% SAV70B} | 97 | ||||||
| Jasione montana | A = {68% SAV, 100% SAV50W, 100% SAV50B} | 86 | 4 | Campanula glomerata | A = {100% SAV, 96% SAV50W, 96% SAV50B} | 89 | 3 |
| B = {32% SAV} | 100 | B = {100% SAV70W, 4% SAV50W} | 97 | ||||
| C = {100% SAV70W} | 100 | C = {100% SAV70B, 4% SAV50B} | 99 | ||||
| D = {100% SAV70B} | 100 | ||||||
| Campanula isophylla | A = {100% SAV, 100% SAV50W, 100% SAV50B, 100% SAV70W} | 97 | 2 | Campanula latifolia | A = {100% SAV, 100% SAV50W, 92% SAV50B} | 99 | 3 |
| B = {100% SAV70B} | 97 | B = {100% SAV70W, 8% SAV50B} | 97 | ||||
| C = {100% SAV70B} | 97 | ||||||
| Campanula rotundifolia | A = {56% SAV, 56% SAV50B, 60% SAV50W} | 88 | 4 | Campanula mollis | A = {84% SAV, 72% SAV50W, 88% SAV50B} | 89 | 3 |
| B = {44% SAV, 44% SAV50B, 40% SAV50W} | 88 | B = {16%SAV, 28% SAV50W, 12% SAV50B} | 86 | ||||
| C = {100% SAV70B} | 100 | C = {100% SAV70B} | 99 | ||||
| D = {100% SAV70W} | 100 | ||||||
| Trachelium caeruleum | A = {100% SAV, 100% SAV50W, 100% SAV50B} | 100 | 3 | Campanula trachelium | A = {100% SAV, 100% SAV50W, 100% SAV50B} | 100 | 3 |
| B = {100% SAV70W} | 97 | B= {100% SAV70W} | 99 | ||||
| C= {100% SAV70B} | 97 | C= {100% SAV70B} | 97 | ||||
| Hesperocodon hederaceus | A = {100% SAV, 100% SAV50B, 100% SAV50W} | 100 | 3 | Campanula versicolor | A = {100% SAV, 100% SAV50W, 100% SAV50B} | 99 | 3 |
| B = {100% SAV70B} | 100 | B = {100% SAV70W} | 97 | ||||
| C = {100% SAV70W} | 100 | C = {100% SAV70B} | 97 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmartín, P.; Gambino, M.; Fuentes, E.; Serrano, M. A Simple, Reliable, and Inexpensive Solution for Contact Color Measurement in Small Plant Samples. Sensors 2020, 20, 2348. https://doi.org/10.3390/s20082348
Sanmartín P, Gambino M, Fuentes E, Serrano M. A Simple, Reliable, and Inexpensive Solution for Contact Color Measurement in Small Plant Samples. Sensors. 2020; 20(8):2348. https://doi.org/10.3390/s20082348
Chicago/Turabian StyleSanmartín, Patricia, Michela Gambino, Elsa Fuentes, and Miguel Serrano. 2020. "A Simple, Reliable, and Inexpensive Solution for Contact Color Measurement in Small Plant Samples" Sensors 20, no. 8: 2348. https://doi.org/10.3390/s20082348
APA StyleSanmartín, P., Gambino, M., Fuentes, E., & Serrano, M. (2020). A Simple, Reliable, and Inexpensive Solution for Contact Color Measurement in Small Plant Samples. Sensors, 20(8), 2348. https://doi.org/10.3390/s20082348