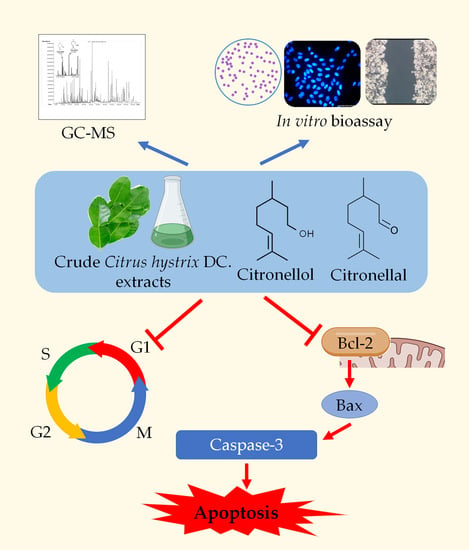
Anticancer Effect of Citrus hystrix DC. Leaf Extract and Its Bioactive Constituents Citronellol and, Citronellal on the Triple Negative Breast Cancer MDA-MB-231 Cell Line
Abstract
:1. Introduction
2. Results
2.1. Extraction Yields of C. hystrix Leaf Powder
2.2. Identification of Volatile Components in Crude Hexane Extract by GC-MS
2.3. Cytotoxicity of Crude Extracts, Citronellol, and Citronellal
2.4. Effect of Crude Hexane, Citronellol, and Citronellal on Cell Proliferation
2.5. Crude Hexane, Citronellol, and Citronellal on Inhibited MDA-MB-231 Cell Migration
2.6. C. hystrix Hexane Extract, Citronellol, and Citronellal Reduced Number of Colonies Forming in MDA-MB-231 Cells
2.7. Crude Hexane, Citronellol, and Citronellal Induced Cell Cycle Arrest in MDA-MB-231 Cell
2.8. Crude Hexane, Citronellol, and Citronellal Induced Apoptosis in MDA-MB-231 Cells
2.9. Crude Hexane, Citronellol, and Citronellal Modulated Apoptosis-Related Proteins Gene Expression in MDA-MB-231 Cells
2.10. Crude Hexane, Citronellol, and Citronellal Induced Apoptosis and DNA Fragmentation in the Cells by Inhibiting the Anti-Apoptotic Bcl-2 Protein and Activating Caspase Dependent Apoptotic Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Materials and Extraction Process
4.3. Cell Culture
4.4. Human Monocyte Isolation
4.5. Gas Chromatography-Mass Spectrometry Analysis (GC-MS)
4.6. Cell Viability MTT Assay
4.7. Identification Effect of Crude Hexane, Citronellol, and Citronellal on Cell Proliferation
4.8. Clonogenic Assay
4.9. Wound Scratch Migration Assay
4.10. Cell Cycle Analysis
4.11. Apoptosis Analysis
4.12. Hoechst 33342 Staining
4.13. RT-qPCR
4.14. Western Blot Analysis
4.15. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| C. hystrix | Citrus hystrix DC. |
| ER | Estrogen receptor |
| GC-MS | Gas chromatography-Mass spectrometry |
| Her-2 | Human epidermal receptor 2 |
| PR | Progesterone receptor |
| RA | Retention areas |
| RI | Retention indices |
| RT | Retention times |
| TNBC | Triple Negative Breast Cancer |
References
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer (Unabridged Version). Arch. Pathol. Lab. Med. 2010, 134, e48–e72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, H., Jr.; Guerra, M.R.; Duarte Cintra, J.R.; Fayer, V.A.; Brum, I.V.; Bustamante Teixeira, M.T. Survival Study of Triple-Negative and Non-Triple-Negative Breast Cancer in a Brazilian Cohort. Clin. Med. Insights Oncol. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Nedeljkovic, M.; Damjanovic, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saklani, A.; Kutty, S.K. Plant-derived compounds in clinical trials. Drug Discov. Today 2008, 13, 161–171. [Google Scholar] [CrossRef]
- Vrignaud, P.; Semiond, D.; Benning, V.; Beys, E.; Bouchard, H.; Gupta, S. Preclinical profile of cabazitaxel. Drug Des. Dev. Ther. 2014, 8, 1851–1867. [Google Scholar] [CrossRef]
- Agouillal, K.; Hagop, M.; O’Brien, S.; Cortes, J. Homoharringtonine/omacetaxine mepesuccinate: The long and winding road to food and drug administration approval. Clin. Lymphoma Myeloma Leuk. 2013, 13, 530–533. [Google Scholar] [CrossRef] [Green Version]
- Agouillal, F.; Taher, Z.M.; Moghrani, H.; Nasrallah, N.; El Enshasy, H. A Review of Genetic Taxonomy, Biomolecules Chemistry and Bioactivities of Citrus hystrix DC. Biosci. Biotechnol. Res. Asia 2017, 14, 285–305. [Google Scholar] [CrossRef]
- Butryee, C.; Sungpuag, P.; Chitchumroonchokchai, C. Effect of processing on the flavonoid content and antioxidant capacity of Citrus hystrix leaf. Int. J. Food Sci. Nutr. 2009, 60, 162–174. [Google Scholar] [CrossRef]
- Dilla Dertyasasa, E.; Anindito Sri Tunjung, W. Volatile Organic Compounds of Kaffir Lime (Citrus hystrix DC.) Leaves Fractions and their Potency as Traditional Medicine. Biosci. Biotechnol. Res. Asia 2017, 14, 1235–1250. [Google Scholar] [CrossRef]
- Salguero, C.P. Kaffir lime. In A Thai Herbal Traditional Recipes for Health and Harmony; Barton, L., Ed.; Findhorn Press: Findhorn Forres, UK, 2003; p. 112. [Google Scholar]
- Tunjung, W.; Cinatl, J.; Michaelis, M.; Mark Smales, C. Anti-Cancer Effect of Kaffir Lime (Citrus Hystrix DC) Leaf Extract in Cervical Cancer and Neuroblastoma Cell Lines. Procedia Chem. 2015, 14, 465–468. [Google Scholar] [CrossRef] [Green Version]
- Utthawang, W.; Ampasavate, W.; Okonogi, S.; Rungrojsakul, M.; Chiampanichayakul, S.; Tima, S.; Anuchapreeda, S. Low doses of partially purified fraction of kaffir lime (Citrus hystrix DC.) leaf extract induce cell death in Molt4 cells. J. Assoc. Med. Sci. 2017, 50, 27–37. [Google Scholar] [CrossRef]
- Sun, S.; Phrutivorapongkul, A.; Dya Fita, D.; Balachandran, C.; Awale, S. Chemical Constituents of Thai Citrus hystrix and Their Antiausterity Activity against the PANC-1 Human Pancreatic Cancer Cell Line. J. Nat. Prod. 2018, 81. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Asano, K.; Sato, T. The Chemical Composition of Citrus Hystrix DC (Swangi). J. Essent. Oil Res. 1990, 2, 179–183. [Google Scholar] [CrossRef]
- Stone, S.; Vasconcellos, F.A.; Lenardao, E.; Do Amaral, R.; Jacob, R.; Leite, F. Evaluation of potential use of Cymbopogon sp. essential oils, (R)-citronellal and N-citronellylamine in cancer chemotherapy. Int. J. Appl. Res. Nat. Prod. 2013, 6, 11–15. [Google Scholar]
- Maßberg, D.; Simon, A.; Häussinger, D.; Keitel, V.; Gisselmann, G.; Conrad, H.; Hatt, H. Monoterpene (−)-citronellal affects hepatocarcinoma cell signaling via an olfactory receptor. Arch. Biochem. Biophys. 2015, 566, 100–109. [Google Scholar] [CrossRef]
- Yu, W.-N.; Lai, Y.-J.; Ma, J.-W.; Ho, C.-T.; Hung, S.-W.; Chen, Y.-H.; Chen, C.-T.; Kao, J.-Y.; Way, T.-D. Citronellol Induces Necroptosis of Human Lung Cancer Cells via TNF-α Pathway and Reactive Oxygen Species Accumulation. In Vivo 2019, 33, 1193–1201. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, N.; Koizumi, M.; Adachi, I.; Kawakami, J. Inhibition of P-glycoprotein-mediated transport by terpenoids contained in herbal medicines and natural products. Food Chem. Toxicol. 2006, 44, 2033–2039. [Google Scholar] [CrossRef]
- Wijayanti, N.; Tunjung, W.; Setyawati, Y. Cytotoxicity and Apoptosis Induction by Kaffir Lime Leaves Extract (Citrus hystrix DC.) In HeLa Cells Culture (Human Cervical Cancer Cell line). KnE Life Sci. 2015, 2, 631. [Google Scholar] [CrossRef] [Green Version]
- Pilco-Ferreto, N.; Calaf, G. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anuchapreeda, S.; Chueahongthong, F.; Viriyaadhammaa, N.; Panyajai, P.; Anzawa, R.; Tima, S.; Ampasavate, C.; Saiai, A.; Rungrojsakul, M.; Usuki, T.; et al. Antileukemic Cell Proliferation of Active Compounds from Kaffir Lime (Citrus hystrix) Leaves. Molecules 2020, 25, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.; Liu, X.Y.; Shi, Y. Citronellol terpenoid inhibits cancer cell proliferation and induces apoptosis in non-small cell lung carcinoma. Lat. Am. J. Pharm. 2015, 34, 1652–1657. [Google Scholar]
- Alenzi, F. Links between apoptosis, proliferation and the cell cycle. Br. J. Biomed. Sci. 2004, 61, 99–102. [Google Scholar] [CrossRef]
- Vandendool, H.; Kratz, P.D. A Generalization Of The Retention Index Sytsem Icluding Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Reed, J.J.; Zenkevich, I.G.; Brown, R.L.; Mallard, W.G.; Stein, S.E. Development of a database of gas chromatographic retention properties of organic compounds. J. Chromatogr. A 2007, 1157, 414–421. [Google Scholar] [CrossRef]
- Linstrom, P.; Mallard, G. The NIST Chemistry WebBook: A chemical data resource on the Internet. J. Chem. Eng. Data 2001, 46. [Google Scholar] [CrossRef]
- Razak, N.A.; Abu, N.; Ho, W.Y.; Zamberi, N.R.; Tan, S.W.; Alitheen, N.B.; Long, K.; Yeap, S.K. Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci. Rep. 2019, 9, 1514. [Google Scholar] [CrossRef]
- Khan, S.A.; Tyagi, M.; Sharma, A.K.; Barreto, S.G.; Sirohi, B.; Ramadwar, M.; Shrikhande, S.V.; Gupta, S. Cell-type specificity of β-actin expression and its clinicopathological correlation in gastric adenocarcinoma. World J. Gastroenterol. 2014, 20, 12202–12211. [Google Scholar] [CrossRef]
- Golestani Eimani, B.; Sanati, M.H.; Houshmand, M.; Ataei, M.; Akbarian, F.; Shakhssalim, N. Expression and prognostic significance of bcl-2 and bax in the progression and clinical outcome of transitional bladder cell carcinoma. Cell J. 2014, 15, 356–363. [Google Scholar]









| No. | RT (min) | R.I 1 | Identified Compounds | Classification | R.A 2 (%) |
|---|---|---|---|---|---|
| 1 | 9.23 | 1100 | Linalool | Monoterpene a | 0.34 |
| 2 | 10.56 | 1146 | Isopulegol | Monoterpene a | 1.01 |
| 3 | 10.75 | 1154 | Citronellal | Monoterpene a | 0.67 |
| 4 | 11.46 | 1179 | Terpinen-4-ol | Monoterpene a | 0.29 |
| 5 | 11.83 | 1193 | α-Terpineol | Monoterpene a | 0.11 |
| 6 | 12.85 | 1229 | Citronellol | Monoterpene a | 1.42 |
| 7 | 14.08 | 1354 | 3,7-dimethyloct-1,7-dien-3,6-diol | Monoterpene a | 0.15 |
| 8 | 16.23 | 1354 | α-Cubebene | Sesquiterpene b | 0.94 |
| 9 | 16.94 | 1381 | α-Copaene | Sesquiterpene b | 1.44 |
| 10 | 17.29 | 1395 | β-Cubebene | Sesquiterpene b | 0.34 |
| 11 | 18.08 | 1426 | Caryophyllene | Sesquiterpene b | 1.59 |
| 12 | 18.30 | 1435 | Bicyclosequiphellandrene | Sesquiterpene b | 0.20 |
| 13 | 18.93 | 1460 | α-Humulene | Sesquiterpene b | 0.23 |
| 14 | 19.47 | 1482 | γ-Muurolene | Sesquiterpene b | 0.12 |
| 15 | 20.05 | 1505 | α-Muurolene | Sesquiterpene b | 0.31 |
| 16 | 20.61 | 1529 | δ-Cadinene | Sesquiterpene b | 0.62 |
| 17 | 21.23 | 1555 | Elemol | Sesquiterpene a | 0.13 |
| 18 | 21.53 | 1568 | Nerolidol | Sesquiterpene a | 0.71 |
| 19 | 21.98 | 1586 | Spathulenol | Sesquiterpene a | 1.34 |
| 20 | 22.12 | 1592 | Caryophyllene oxide | Sesquiterpene a | 3.74 |
| 21 | 23.21 | 1641 | Caryophylladienol | Sesquiterpene a | 0.39 |
| 22 | 23.67 | 1661 | Viridiflorene | Sesquiterpene b | 0.20 |
| 23 | 24.05 | 1678 | Caryophyllenol | Sesquiterpene a | 1.14 |
| 24 | 25.90 | 1764 | Tetradecanoic acid | Fatty acid a | 0.34 |
| 25 | 26.29 | 1785 | Alloaromadendrene oxide | Sesquiterpene a | 0.22 |
| 26 | 27.63 | 1847 | Hexahydrofarnesyl acetone | Sesquiterpene derivative | 0.81 |
| 27 | 29.24 | 1927 | Methyl palmitate | Fatty acid a | 0.30 |
| 28 | 30.19 | 1976 | Palmitic acid | Fatty acid a | 6.82 |
| 29 | 32.79 | 2015 | Phytol | Diterpene | 0.40 |
| 30 | 33.30 | 2144 | Linoleic acid | Fatty acid a | 1.89 |
| 31 | 33.40 | 2149 | (6Z),(9Z)-Pentadecadien-1-ol | Fatty acid a | 2.39 |
| 32 | 44.09 | 2833 | Supraene | Triterpene | 0.31 |
| 33 | 44.92 | 2893 | cis-2,6-Dimethyl-2,6-octadiene | Monoterpene b | 2.19 |
| 34 | 48.24 | 3103 | Tetracosane | Hydrocarbon | 3.21 |
| 35 | 48.98 | 3139 | α-Tocopherol | Vitamin | 0.56 |
| 36 | 49.15 | 3147 | Pentacosane | Hydrocarbon | 1.03 |
| 37 | 50.92 | 3227 | Campesterol | Phytosterol | 0.46 |
| 38 | 51.73 | 3260 | Stigmasterol | Phytosterol | 1.07 |
| 39 | 52.86 | 3309 | Heneicosane | Hydrocarbon | 2.57 |
| 40 | 53.00 | 3350 | 1-Eicosanol | Fatty alcohol | 0.37 |
| 41 | 53.27 | 3317 | γ-Sitosterol | Phytosterol | 2.90 |
| 42 | 53.81 | 3335 | Lanosterol | Triterpene | 2.45 |
| 43 | 58.99 | 3485 | Lupenyl acetate | Triterpene | 0.68 |
| 44 | 60.02 | 3510 | 17-Pentriacontene | Hydrocarbon | 2.23 |
| 45 | 62.13 | 3556 | Neophytadiene | Diterpene b | 0.61 |
| Total R.A of identified compounds | 51.24% | ||||
| Oxygenated monoterpenes | 3.99% | ||||
| Hydrocarbon monoterpene | 2.19% | ||||
| Oxygenated sesquiterpenes | 7.67% | ||||
| Hydrocarbon sesquiterpenes | 5.99% | ||||
| Hydrocarbons | 9.04% | ||||
| Fatty acids and fatty alcohols | 12.11% | ||||
| Other | 10.25% | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, Y.; Suphrom, N.; Daowtak, K.; Potup, P.; Thongsri, Y.; Usuwanthim, K. Anticancer Effect of Citrus hystrix DC. Leaf Extract and Its Bioactive Constituents Citronellol and, Citronellal on the Triple Negative Breast Cancer MDA-MB-231 Cell Line. Pharmaceuticals 2020, 13, 476. https://doi.org/10.3390/ph13120476
Ho Y, Suphrom N, Daowtak K, Potup P, Thongsri Y, Usuwanthim K. Anticancer Effect of Citrus hystrix DC. Leaf Extract and Its Bioactive Constituents Citronellol and, Citronellal on the Triple Negative Breast Cancer MDA-MB-231 Cell Line. Pharmaceuticals. 2020; 13(12):476. https://doi.org/10.3390/ph13120476
Chicago/Turabian StyleHo, Yathsoeung, Nungruthai Suphrom, Krai Daowtak, Pachuen Potup, Yordhathai Thongsri, and Kanchana Usuwanthim. 2020. "Anticancer Effect of Citrus hystrix DC. Leaf Extract and Its Bioactive Constituents Citronellol and, Citronellal on the Triple Negative Breast Cancer MDA-MB-231 Cell Line" Pharmaceuticals 13, no. 12: 476. https://doi.org/10.3390/ph13120476
APA StyleHo, Y., Suphrom, N., Daowtak, K., Potup, P., Thongsri, Y., & Usuwanthim, K. (2020). Anticancer Effect of Citrus hystrix DC. Leaf Extract and Its Bioactive Constituents Citronellol and, Citronellal on the Triple Negative Breast Cancer MDA-MB-231 Cell Line. Pharmaceuticals, 13(12), 476. https://doi.org/10.3390/ph13120476