Comparison of Chemical Composition and Biological Activities of Eight Selaginella Species
Abstract
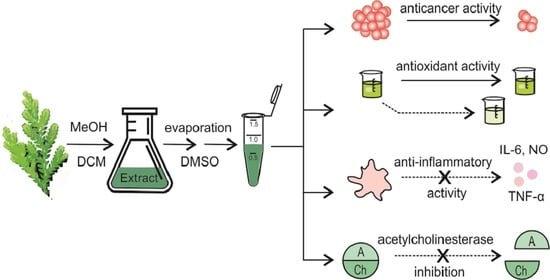
:1. Introduction
2. Results
2.1. Chemical Composition
2.2. Antioxidant Activity
2.3. Cytotoxicity against Cancer Cell Lines
2.4. Inhibition of Acetylcholinesterase Activity
2.5. Anti-Inflammatory Activity
3. Discussion
4. Materials and Methods
4.1. Material and Reagents
4.2. Plant Material
4.3. Extraction of Secondary Metabolites
4.4. Determination and Identification of Secondary Metabolites
4.5. Oxygen Radical Absorption Capacity (ORAC)
4.6. Cytotoxicity Assay
4.7. Modulation of Acetylcholinesterase Activity
4.8. Anti-Inflammatory Activity
4.9. Statistical Analysis and Correlation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weng, J.-K.; Noel, J. Chemodiversity in selaginella: A reference system for parallel and convergent metabolic evolution in terrestrial plants. Front. Plant Sci. 2013, 4, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, P.H.; Zhao, B.T.; Ali, M.Y.; Choi, J.S.; Rhyu, D.Y.; Min, B.S.; Woo, M.H. Insulin-mimetic selaginellins from selaginella tamariscina with protein tyrosine phosphatase 1B (PTP1B) inhibitory activity. J. Nat. Prod. 2015, 78, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Ji, D.J.; Han, Y.R.; Choi, J.S.; Rhyu, D.Y.; Min, B.S.; Woo, M.H. Selaginellin and biflavonoids as protein tyrosine phosphatase 1b inhibitors from Selaginella tamariscina and their glucose uptake stimulatory effects. Bioorg. Med. Chem. 2015, 23, 3730–3737. [Google Scholar] [CrossRef] [PubMed]
- Adame-González, A.B.; Muñíz-Dl, M.E.; Valencia, -A.S. Comparative leaf morphology and anatomy of six Selaginella species (selaginellaceae, subgen, rupestrae) with notes on xerophytic adaptations. Flora 2019, 260, 151482. [Google Scholar] [CrossRef]
- Uphof, J.C.T. Physiological anatomy of xerophytic Selaginellas. New Phytol. 1920, 19, 101–131. [Google Scholar] [CrossRef]
- Weststrand, S.; Korall, P. Phylogeny of Selaginellaceae: There is value in morphology after all! Am. J. Bot. 2016, 103, 2136–2159. [Google Scholar] [CrossRef] [Green Version]
- Magazù, S.; Migliardo, F.; Benedetto, A.; La Torre, R.; Hennet, L. Bio-protective effects of homologous disaccharides on biological macromolecules. Eur. Biophys. J. 2012, 41, 361–367. [Google Scholar] [CrossRef]
- Schulz, C.; Little, D.P.; Stevenson, D.W.; Bauer, D.; Moloney, C.; Stützel, T. An overview of the morphology, anatomy, and life cycle of a new model species: The lycophyte Selaginella apoda (l.) spring. Int. J. Plant Sci. 2010, 171, 693–712. [Google Scholar] [CrossRef]
- Heo, J.K.; Nguyen, P.H.; Kim, W.C.; Phuc, N.M.; Liu, K.H. Inhibitory effect of selaginellins from Selaginella tamariscina (beauv.) spring against cytochrome P450 and uridine 5’-diphosphoglucuronosyltransferase isoforms on human liver microsomes. Molecules 2017, 22, 1590. [Google Scholar] [CrossRef] [Green Version]
- Shim, S.Y.; Lee, S.G.; Lee, M. Biflavonoids isolated from Selaginella tamariscina and their anti-inflammatory activities via ERK 1/2 signaling. Molecules 2018, 23, 926. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Ma, X.; Li, D.; Jiang, Y.; Liang, Q.; Hui, C. Chemical composition of essential volatile oils of Selaginella spp. and their antibacterial activity. Bangladesh J. Bot. 2018, 47, 719–726. [Google Scholar]
- Chandran, G.; Muralidhara. Neuroprotective effect of aqueous extract of Selaginella delicatula as evidenced by abrogation of rotenone-induced motor deficits, oxidative dysfunctions, and neurotoxicity in mice. Cell Mol. Neurobiol. 2013, 33, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Girish, C.; Muralidhara. Propensity of Selaginella delicatula aqueous extract to offset rotenone-induced oxidative dysfunctions and neurotoxicity in drosophila melanogaster: Implications for parkinson’s disease. NeuroToxicology 2012, 33, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Duh, C.Y.; Chen, J.F. New cytotoxic biflavonoids from Selaginella delicatula. Planta medica 2005, 71, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Q.; Deng, R.; Zhang, S.; Lu, Y. Transcriptome profiling of blue leaf coloration in Selaginella uncinata. Canadian J. Plant Sci. 2016, 97. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lei, X.; Chen, K.L. Comparison of cytotoxic activities of extracts from Selaginella species. Pharmacogn. Mag. 2014, 10, 529–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Zheng, Y.; Zhi, H.; Dai, Y.; Wang, N.-L.; Fang, Y.-X.; Du, Z.-Y.; Zhang, K.; Wu, L.-Y.; Fan, M. γ-Lactone derivatives and terpenoids from Selaginella uncinata and their protective effect against anoxia. Chem. Nat. Compd. 2014, 50, 366–369. [Google Scholar] [CrossRef]
- Yu, B.; Cai, W.; Zhang, H.H.; Zhong, Y.S.; Fang, J.; Zhang, W.Y.; Mo, L.; Wang, L.C.; Yu, C.H. Selaginella uncinata flavonoids ameliorated ovalbumin-induced airway inflammation in a rat model of asthma. J. Ethnopharmacol. 2017, 195, 71–80. [Google Scholar] [CrossRef]
- Zou, Z.X.; Xu, P.S.; Zhang, G.G.; Cheng, F.; Chen, K.; Li, J.; Zhu, W.X.; Cao, D.S.; Xu, K.P.; Tan, G.S. Selagintriflavonoids with BACE1 inhibitory activity from the fern Selaginella doederleinii. Phytochemistry 2017, 134, 114–121. [Google Scholar] [CrossRef]
- Zou, Z.X.; Xu, K.P.; Xu, P.S.; Li, X.M.; Cheng, F.; Li, J.; Yu, X.; Cao, D.S.; Li, D.; Zeng, W.; et al. Seladoeflavones A–F, six novel flavonoids from Selaginella doederleinii. Fitoterapia 2017, 116, 66–71. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, R.Z.; Wang, C.G.; Ouyang, X.L.; Jing, X.T.; Liang, D.; Wang, H.S. New inhibitors of matrix metalloproteinases 9 (MMP-9): Lignans from Selaginella moellendorffii. Fitoterapia 2018, 130, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Xu, K.P.; Liu, L.F.; Yao, C.P.; Xu, P.S.; Zhou, G.; Li, D.; Li, X.M.; Chen, K.; Zou, Z.X.; et al. New neolignans from Selaginella pieta and their protective effect on HT-22 cells. Fitoterapia 2018, 127, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Long, C.-L.; Yang, F.-M.; Wang, X.; Sun, Q.-Y.; Wang, H.-S.; Shi, Y.-N.; Tang, G.-H. Pyrrolidinoindoline alkaloids from Selaginella moellendorfii. J. Nat. Prod. 2009, 72, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.Y.; Zhang, Y.; Xia, M.Y.; Zhuo, J.X.; Wang, Y.H.; Long, C.L. Modified abietane diterpenoids from whole plants of Selaginella moellendorffii. J. Nat. Prod. 2018, 81, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qian, Y.; Tian, Y.J.; Yuan, S.M.; Wei, W.; Wang, G. Optimization of ionic liquid-assisted extraction of biflavonoids from Selaginella doederleinii and evaluation of its antioxidant and antitumor activity. Molecules 2017, 22, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Li, D.; Ma, X.; Jiang, F.; He, Q.; Qiu, S.; Li, Y.; Wang, G. Ionic liquid(-)ultrasound-based extraction of biflavonoids from Selaginella helvetica and investigation of their antioxidant activity. Molecules 2018, 23, 3284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Zhao, M.; Zhu, Y.; Zhu, Z.H.; Oberer, L.; Duan, J.A. Diselaginellin b, an unusual dimeric molecule from Selaginella pulvinata, inhibited metastasis and induced apoptosis of smmc-7721 human hepatocellular carcinoma cells. J. Nat. Prod. 2017, 80, 3151–3158. [Google Scholar] [CrossRef]
- Zou, Z.; Xu, P.; Wu, C.; Zhu, W.; Zhu, G.; He, X.; Zhang, G.; Hu, J.; Liu, S.; Zeng, W.; et al. Carboxymethyl flavonoids and a chromone with antimicrobial activity from Selaginella moellendorffii hieron. Fitoterapia 2016, 111, 124–129. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.J.; Tan, N.H.; Oberer, L.; Wagner, T.; Wu, Y.P.; Zeng, G.Z.; Yan, H.; Wang, Q. Antimicrobial selaginellin derivatives from Selaginella pulvinata. Bioorg. Med. Chem. Lett. 2010, 20, 2456–2460. [Google Scholar] [CrossRef]
- Cao, Y.; Yao, Y.; Huang, X.-J.; Oberer, L.; Wagner, T.; Guo, J.-M.; Gu, W.; Liu, W.-D.; Lv, G.-X.; Shen, Y.-N.; et al. Four new selaginellin derivatives from Selaginella pulvinata: Mechanism of racemization process in selaginellins with quinone methide. Tetrahedron 2015, 71, 1581–1587. [Google Scholar] [CrossRef]
- Zhang, L.-P.; Liang, Y.-M.; Wei, X.-C.; Cheng, D.-L. A new unusual natural pigment from Selaginella sinensis and its noticeable physicochemical properties. J. Org. Chem. 2007, 72, 3921–3924. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.L.; Ma, S.C.; Yu, J.D.; Yang, S.Y.; Xiao, X.Y.; Hu, J.Y.; Lu, Y.; Shaw, P.C.; But, P.P.; Lin, R.C. Selaginellin A and B, two novel natural pigments isolated from Selaginella tamariscina. Chem. Pharm. Bull. 2008, 56, 982–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Shao, Y.; Li, K.; Xia, W. Bioactive selaginellins from Selaginella tamariscina (beauv.) spring. Beilstein J. Org. Chem. 2012, 8, 1884–1889. [Google Scholar] [CrossRef] [PubMed]
- Scifinder. Available online: https://scifinder-n.cas.org/ (accessed on 31 July 2020).
- Zhang, G.G.; Jing, Y.; Zhang, H.M.; Ma, E.L.; Guan, J.; Xue, F.N.; Liu, H.X.; Sun, X.Y. Isolation and cytotoxic activity of selaginellin derivatives and biflavonoids from Selaginella tamariscina. Planta Med. 2012, 78, 390–392. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.; Kang, K.B.; Kim, J.; Sung, S.H. Molecular networking reveals the chemical diversity of selaginellin derivatives, natural phosphodiesterase-4 inhibitors from Selaginella tamariscina. J. Nat. Prod. 2019, 82, 1820–1830. [Google Scholar] [CrossRef]
- Le, D.D.; Nguyen, D.H.; Zhao, B.T.; Seong, S.H.; Choi, J.S.; Kim, S.K.; Kim, J.A.; Min, B.S.; Woo, M.H. Ptp1b inhibitors from selaginella tamariscina (beauv.) spring and their kinetic properties and molecular docking simulation. Bioorg. Chem. 2017, 72, 273–281. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.-J.; Tan, N.-H.; Wu, Y.-P.; Yang, J.; Wang, Q. Structure determination of selaginellins G and H from Selaginella pulvinata by NMR spectroscopy. Magn. Reson. Chem. 2010, 48, 656–659. [Google Scholar] [CrossRef]
- Zálešák, F.; Bon, D.J.D.; Pospíšil, J. Lignans and neolignans: Plant secondary metabolites as a reservoir of biologically active substances. Pharmacol. Res. 2019, 146, 104284. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, T.-B.; Hou, L.-J.; Lv, H.-X.; Liu, A.M.; Zeng, P.; Li, A.-H. A new selaginellin from Selaginella moellendorffii inhibits hepatitis B virus gene expression and replication. Chem. Nat. Compd. 2016, 52, 624–627. [Google Scholar] [CrossRef]
- Liu, X.; Luo, H.-B.; Huang, Y.-Y.; Bao, J.-M.; Tang, G.-H.; Chen, Y.-Y.; Wang, J.; Yin, S. Selaginpulvilins A–D, new phosphodiesterase-4 inhibitors with an unprecedented skeleton from Selaginella pulvinata. Org. Lett. 2014, 16, 282–285. [Google Scholar] [CrossRef]
- Yao, W.-N.; Huang, R.-Z.; Hua, J.; Zhang, B.; Wang, C.-G.; Liang, D.; Wang, H.-S. Selagintamarlin a: A selaginellin analogue possessing a 1H-2-benzopyran core from Selaginella tamariscina. ACS Omega 2017, 2, 2178–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.-S.; Liu, X.; Weng, J.; Guo, Y.-Q.; Li, Q.-J.; Ahmed, A.; Tang, G.-H.; Yin, S. Natural diarylfluorene derivatives: Isolation, total synthesis, and phosphodiesterase-4 inhibition. Org. Chem. Front. 2017, 4, 170–177. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Wu, D.; Tang, G.; Lai, Z.; Zheng, X.; Yin, S.; Luo, H.-B. The discovery, complex crystal structure, and recognition mechanism of a novel natural PDE4 inhibitor from Selaginella pulvinata. Biochem. Pharmacol. 2017, 130, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Luong, T.N.; Lepik, M.; Aftab, N.; Fong, V.H.; Vieira, A. Synergism of antioxidant phytochemicals: Comparisons among purified polyphenols and dietary-plant extracts, XXVIII International Horticultural Congress, 2012; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2012; pp. 121–127. [Google Scholar] [CrossRef]
- Macêdo, L.A.R.d.O.; Oliveira Júnior, R.G.d.; Souza, G.R.; de Oliveira, A.P.; de Lavor, É.M.; Silva, M.G.e.; Pacheco, A.G.M.; de Menezes, I.R.A.; Coutinho, H.D.M.; Pessoa, C.d.Ó.; et al. Chemical composition, antioxidant and antibacterial activities and evaluation of cytotoxicity of the fractions obtained from Selaginella convoluta (arn.) spring (Selaginellaceae). Biotechnol. Equip. 2018, 32, 506–512. [Google Scholar] [CrossRef] [Green Version]
- Rao, L.; You, Y.-X.; Su, Y.; Fan, Y.; Liu, Y.; He, Q.; Chen, Y.; Meng, J.; Hu, L.; Li, Y.; et al. Lignans and neolignans with antioxidant and human cancer cell proliferation inhibitory activities from Cinnamomum bejolghota confirm its functional food property. J. Agric. Food Chem. 2020, 68, 8825–8835. [Google Scholar] [CrossRef]
- Singh, K.; Gangrade, A.; Jana, A.; Mandal, B.B.; Das, N. Design, synthesis, characterization, and antiproliferative activity of organoplatinum compounds bearing a 1,2,3-triazole ring. ACS Omega 2019, 4, 835–841. [Google Scholar] [CrossRef]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar]
- Perreault, M.; Maltais, R.; Dutour, R.; Poirier, D. Explorative study on the anticancer activity, selectivity and metabolic stability of related analogs of aminosteroid RM-133. Steroids 2016, 115, 105–113. [Google Scholar] [CrossRef]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on breast cancer cell lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef]
- Qian, Z.; Cai-xia, W.; Yan-ling, L.; Cui-yan, L.; Yan-hua, R. Chemical constituents from Selaginella doederleinii and their bioactivities. Zhongcaoyao 2013, 44, 3270–3275. [Google Scholar]
- Kathirvel, P.; Ravi, S. Chemical composition of the essential oil from basil (Ocimum basilicum linn.) and its in vitro cytotoxicity against HeLa and HEP-2 human cancer cell lines and nih 3t3 mouse embryonic fibroblasts. Nat. Prod. Res. 2012, 26, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Zou, Z.X.; Xu, P.S.; Zhang, S.H.; Zhang, Y.; Yao, C.P.; Xu, K.P.; Tan, G.S. Pictalignans D–F, three new neolignan derivatives from Selaginella picta. Nat. Prod. Res. 2020, 34, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, S.E.; Knyazeva, E.L.; Khasanova, L.M.; Fadeev, R.S.; Zhadan, A.P.; Roche-Hakansson, H.; Håkansson, A.P.; Akatov, V.S.; Permyakov, E.A. Oleic acid is a key cytotoxic component of hamlet-like complexes. Biol. Chem. 2012, 393, 85. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Barreyro, F.J.; Isomoto, H.; Bronk, S.F.; Gores, G.J. Free fatty acids sensitise hepatocytes to trail mediated cytotoxicity. Gut 2007, 56, 1124–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Li, C.Y.; Chiu, C.C.; Niwa, M.; Kitanaka, S.; Damu, A.G.; Lee, E.J.; Wu, T.S. Constituents of leaves of Phellodendron japonicum maxim. and their antioxidant activity. Chem. Pharm. Bull. 2005, 53, 1118–1121. [Google Scholar] [CrossRef] [Green Version]
- Paswan, S.K.; Gautam, A.; Verma, P.; Rao, C.V.; Sidhu, O.P.; Singh, A.P.; Srivastava, S. The indian magical herb ‘sanjeevni’ (Selaginella bryopteris l.)—A promising anti-inflammatory phytomedicine for the treatment of patients with inflammatory skin diseases. J. Pharmacopunct. 2017, 20, 93–99. [Google Scholar] [CrossRef]
- Gayathri, V.; Asha, V.V.; John, J.A.; Subramoniam, A. Protection of immunocompromised mice from fungal infection with a thymus growth-stimulatory component from Selaginella involvens, a fern. Immunopharmacol. Immunotoxicol. 2011, 33, 351–359. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J.Y.; Park, J.H.; Jung, H.S.; Kim, J.S.; Kang, S.S.; Kim, Y.S.; Han, Y. Immunoregulatory activity by daucosterol, a β-sitosterol glycoside, induces protective th1 immune response against disseminated candidiasis in mice. Vaccine 2007, 25, 3834–3840. [Google Scholar] [CrossRef]
- Kim, M.J.; Wang, H.S.; Lee, M.W. Anti-inflammatory effects of fermented bark of Acanthopanax sessiliflorus and its isolated compounds on lipopolysaccharide-treated RAW 264.7 macrophage cells. Evid. Based Complement Alternat. Med. 2020, 2020, 6749425. [Google Scholar] [CrossRef]
- Jang, J.; Kim, S.-M.; Yee, S.-M.; Kim, E.-M.; Lee, E.-H.; Choi, H.-R.; Lee, Y.-S.; Yang, W.-K.; Kim, H.-Y.; Kim, K.-H.; et al. Daucosterol suppresses dextran sulfate sodium (dss)-induced colitis in mice. Int. Immunopharmacol. 2019, 72, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, Y.; Cheng, Z.; Hu, Y.; Liu, T. Sotetsuflavone suppresses invasion and metastasis in non-small-cell lung cancer a549 cells by reversing EMT via the TNF-α/NF-κB and PI3K/AKT signaling pathway. Cell Death Discov. 2018, 4, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Viktorova, J.; Stranska-Zachariasova, M.; Fenclova, M.; Vitek, L.; Hajslova, J.; Kren, V.; Ruml, T. Complex evaluation of antioxidant capacity of milk thistle dietary supplements. Antioxidants 2019, 8, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aatbio EC50 calculator. Available online: https://www.aatbio.com/tools/ec50-calculator/ (accessed on 31 September 2020).
- Tran, V.N.; Viktorova, J.; Augustynkova, K.; Jelenova, N.; Dobiasova, S.; Rehorova, K.; Fenclova, M.; Stranska-Zachariasova, M.; Vitek, L.; Hajslova, J.; et al. In silico and in vitro studies of mycotoxins and their cocktails; their toxicity and its mitigation by silibinin pre-treatment. Toxins 2020, 12, 148. [Google Scholar] [CrossRef] [Green Version]
- Dobiasová, S.; Řehořová, K.; Kučerová, D.; Biedermann, D.; Káňová, K.; Petrásková, L.; Koucká, K.; Václavíková, R.; Valentová, K.; Ruml, T.; et al. Multidrug resistance modulation activity of silybin derivatives and their anti-inflammatory potential. Antioxidants 2020, 9, 455. [Google Scholar] [CrossRef]






| Metabolites | S. apoda | S. biformis | S. cupressina | S. delicatula | S. erythropus | S. myosuroides | S. uncinata | S. ramosii |
|---|---|---|---|---|---|---|---|---|
| Alkaloids | 2 | 2 | 1 | 4 | 1 | 0 | 2 | 2 |
| Fatty acids | 3 | 1 | 2 | 3 | 3 | 3 | 2 | 2 |
| Flavonoids | 36 | 37 | 27 | 33 | 35 | 25 | 32 | 29 |
| Lignans/neolignans | 20 | 15 | 7 | 9 | 16 | 10 | 15 | 6 |
| Phenols | 19 | 15 | 11 | 14 | 11 | 11 | 11 | 12 |
| Quinones | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Selaginellins | 13 | 12 | 7 | 8 | 7 | 4 | 11 | 8 |
| Steroids | 4 | 3 | 2 | 3 | 1 | 0 | 3 | 2 |
| Terpenoids | 1 | 1 | 2 | 2 | 2 | 2 | 3 | 2 |
| Unidentified | 3 | 2 | 1 | 1 | 2 | 2 | 2 | 2 |
| Total | 102 | 89 | 61 | 78 | 79 | 58 | 81 | 66 |
| Metabolites | S. apoda | S. biformis | S. cupressina | S. delicatula | S. erythropus | S. myosuroides | S. uncinata | S. ramosii |
|---|---|---|---|---|---|---|---|---|
| Fatty acids | 2 | 1 | 1 | 1 | 2 | 3 | 2 | 2 |
| Flavonoids | 3 | 5 | 4 | 5 | 7 | 7 | 7 | 7 |
| Lignans/neolignans | 1 | 1 | 5 | 5 | 6 | 7 | 5 | 5 |
| Phenols | 3 | 4 | 3 | 4 | 6 | 7 | 4 | 7 |
| Quinones | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Selaginellins | 1 | 3 | 3 | 3 | 5 | 4 | 4 | 5 |
| Steroids | 2 | 2 | 0 | 0 | 4 | 3 | 2 | 5 |
| Terpenoids | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Unidentified | 2 | 6 | 2 | 1 | 10 | 14 | 12 | 13 |
| Total | 15 | 23 | 19 | 20 | 41 | 46 | 37 | 46 |
| Value | n | df | Crit. Value | α | |
|---|---|---|---|---|---|
| HeLa | |||||
| 5,5′′,7,7′′,4′,4′′′-Hexahydroxy-(2′,6′′)-biflavone or 5,5′′,7,7′′,4′,4′′′-Hexahydroxy-(2′,8′′)-biflavone | 0.759 | 7 | 5 | 0.754 | 0.05 |
| Selaginpulvilin F or selaginpulvilin K or selaginellin Q | 0.759 | 7 | 5 | 0.754 | 0.05 |
| Selaginellin O or selaginpulvilin B | 0.999 | 3 | 1 | 0.997 | 0.05 |
| Selaginpulvilin E or selaginpulvilin L or selaginellin P | 0.999 | 3 | 1 | 0.997 | 0.05 |
| Selaginpulvilin I or selaginellin W | 0.997 | 3 | 1 | 0.997 | 0.05 |
| SI (HaCat/HeLa) | |||||
| Seladoeflavone E | 0.982 | 4 | 2 | 0.980 | 0.02 |
| Sinensioside A | 1.000 | 3 | 1 | 1.000 | 0.02 |
| SI (HEK 293T/HepG2) | |||||
| Pinocembrin-7-O-β-d-glucopyranoside | 0.733 | 8 | 6 | 0.707 | 0.05 |
| Compound 1 * | 0.733 | 8 | 6 | 0.707 | 0.05 |
| Methyl cinnamate | 0.733 | 8 | 6 | 0.707 | 0.05 |
| Compound 2 * | 0.895 | 6 | 4 | 0.882 | 0.02 |
| Oleic acid | 0.892 | 5 | 3 | 0.878 | 0.05 |
| Chalcone | 0.864 | 8 | 6 | 0.834 | 0.01 |
| 5-Carbomethoxymethyl-4′,7-dihydroxyflavone | 0.814 | 6 | 4 | 0.811 | 0.05 |
| Phellodensin F | 0.774 | 8 | 6 | 0.707 | 0.05 |
| Compound 3 * | 0.789 | 8 | 6 | 0.789 | 0.02 |
| Viburnolide C | 0.774 | 8 | 6 | 0.707 | 0.05 |
| 1-Methoxy-3-methylanthraquinone | 0.789 | 8 | 6 | 0.789 | 0.02 |
| Compound 4 * | 0.998 | 3 | 1 | 0.997 | 0.02 |
| HEK 293T | |||||
| Compound 5 * | 0.999 | 3 | 1 | 0.997 | 0.05 |
| Value | n | df | Crit. Value | α | |
|---|---|---|---|---|---|
| NO production | |||||
| Lignans or neolignans [39] | 0.856 | 8 | 6 | 0.834 | 0.01 |
| Seladoeflavone E | 0.920 | 5 | 3 | 0.878 | 0.05 |
| Selamoellenin A | 0.920 | 5 | 3 | 0.878 | 0.05 |
| TNF-α production | |||||
| Robustaflavone 4′-methyl ether or podocarpusflavone or neocryptomerin or sequoiaflavoneor isocryptomerin or sotetsuflavone | 0.989 | 5 | 3 | 0.934 | 0.02 |
| Selagin | 0.908 | 6 | 4 | 0.811 | 0.05 |
| 3-Hydroxy-4-carboxy-2-methoxyphenyl ester benzenepropanoic acid | 0.908 | 6 | 4 | 0.811 | 0.05 |
| Pulvinataphendiol | 0.908 | 6 | 4 | 0.811 | 0.05 |
| IL-6 production | |||||
| Selagin | 0.943 | 6 | 4 | 0.917 | 0.01 |
| 3-Hydroxy-4-carboxy-2-methoxyphenyl ester benzenepropanoic acid | 0.943 | 6 | 4 | 0.917 | 0.01 |
| Pulvinataphendiol | 0.943 | 6 | 4 | 0.917 | 0.01 |
| Daucosterol | 0.813 | 6 | 4 | 0.811 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Křížkovská, B.; Kumar, R.; Řehořová, K.; Sýkora, D.; Dobiasová, S.; Kučerová, D.; Tan, M.C.; Linis, V.; Oyong, G.; Ruml, T.; et al. Comparison of Chemical Composition and Biological Activities of Eight Selaginella Species. Pharmaceuticals 2021, 14, 16. https://doi.org/10.3390/ph14010016
Křížkovská B, Kumar R, Řehořová K, Sýkora D, Dobiasová S, Kučerová D, Tan MC, Linis V, Oyong G, Ruml T, et al. Comparison of Chemical Composition and Biological Activities of Eight Selaginella Species. Pharmaceuticals. 2021; 14(1):16. https://doi.org/10.3390/ph14010016
Chicago/Turabian StyleKřížkovská, Bára, Rohitesh Kumar, Kateřina Řehořová, David Sýkora, Simona Dobiasová, Denisa Kučerová, Maria Carmen Tan, Virgilio Linis, Glenn Oyong, Tomáš Ruml, and et al. 2021. "Comparison of Chemical Composition and Biological Activities of Eight Selaginella Species" Pharmaceuticals 14, no. 1: 16. https://doi.org/10.3390/ph14010016
APA StyleKřížkovská, B., Kumar, R., Řehořová, K., Sýkora, D., Dobiasová, S., Kučerová, D., Tan, M. C., Linis, V., Oyong, G., Ruml, T., Lipov, J., & Viktorová, J. (2021). Comparison of Chemical Composition and Biological Activities of Eight Selaginella Species. Pharmaceuticals, 14(1), 16. https://doi.org/10.3390/ph14010016