Clinical Outcomes of Secondary Prophylactic Granulocyte Colony-Stimulating Factors in Breast Cancer Patients at a Risk of Neutropenia with Doxorubicin and Cyclophosphamide-Based Chemotherapy
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
2.2. The Overall Incidence of Severe Neutropenic Events and Risk Factors
2.3. Recurrence of Severe Neutropenic Events and Its Risk Factors
2.4. RDI after Experiencing Severe Neutropenic Events
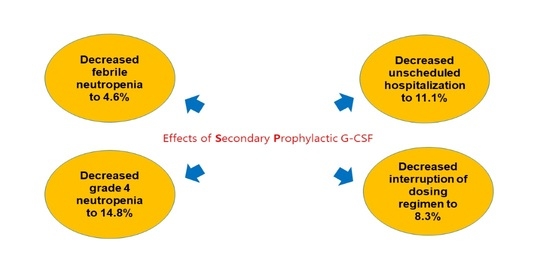
2.5. Clinical Outcomes of Secondary Prophylaxis of G-CSF
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Study Design
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berry, D.A.; Cronin, K.A.; Feuer, E.J.; Plevritis, S.K.; Fryback, D.G.; Clarke, L.; Zelen, M.; Mandelblatt, J.S.; Yakovlev, A.Y.; Habbema, J.D.F. Effect of Screening and Adjuvant Therapy on Mortality from Breast Cancer. N. Engl. J. Med. 2005, 353, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of Chemotherapy and Hormonal Therapy for Early Breast Cancer on Recurrence and 15-Year Survival: An Overview of the Randomised Trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- Fujii, T.; Le Du, F.; Xiao, L.; Kogawa, T.; Barcenas, C.H.; Alvarez, R.H.; Valero, V.; Shen, Y.; Ueno, N.T. Effectiveness of an Adjuvant Chemotherapy Regimen for Early-Stage Breast Cancer: A Systematic Review and Network Meta-Analysis. JAMA Oncol. 2015, 1, 1311–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyman, G.H.; Dale, D.C.; Crawford, J. Incidence and Predictors of Low Dose-Intensity in Adjuvant Breast Cancer Chemotherapy: A Nationwide Study of Community Practices. J. Clin. Oncol. 2003, 21, 4524–4531. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, S.Y.; Kim, J.W.; Choi, Y.J.; Seo, J.H.; Shin, S.W.; Kim, Y.H.; Kim, J.S.; Park, K.H.; Park, I.H.; et al. Incidence and Predictors of Febrile Neutropenia among Early-Stage Breast Cancer Patients Receiving Anthracycline-Based Chemotherapy in Korea. Oncology 2016, 91, 274–282. [Google Scholar] [CrossRef]
- Kim, C.G.; Sohn, J.; Chon, H.; Kim, J.H.; Heo, S.J.; Cho, H.; Kim, I.J.; Kim, G.M.; Kim, S.I.; Park, S.; et al. Incidence of Febrile Neutropenia in Korean Female Breast Cancer Patients Receiving Preoperative or Postoperative Doxorubicin/Cyclophosphamide Followed by Docetaxel Chemotherapy. J. Breast Cancer 2016, 19, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Von Minckwitz, G.; Raab, G.; Caputo, A.; Schütte, M.; Hilfrich, J.; Blohmer, J.U.; Gerber, B.; Costa, S.D.; Merkle, E.; Eidtmann, H.; et al. Doxorubicin with Cyclophosphamide Followed by Docetaxel Every 21 Days Compared with Doxorubicin and Docetaxel Every 14 Days as Preoperative Treatment in Operable Breast Cancer: The GEPARDUO Study of the German Breast Group. J. Clin. Oncol. 2005, 23, 2676–2685. [Google Scholar] [CrossRef]
- Chan, A.; Chen, C.; Chiang, J.; Tan, S.H.; Ng, R. Incidence of Febrile Neutropenia among Early-Stage Breast Cancer Patients Receiving Anthracycline-Based Chemotherapy. Support Care Cancer 2012, 20, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Aryal, M.R.; Karmacharya, P.; Giri, S.; Martin, M.G.; Bhatt, V.R. Mortality, Length of Stay, and Health Care Costs of Febrile Neutropenia-Related Hospitalizations among Patients with Breast Cancer in the United States. Support Care Cancer 2015, 23, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Lynn, J.; Chen, K.; Weng, Y.; Chiu, T. Risk Factors Associated with Complications in Patients with Chemotherapy-Induced Febrile Neutropenia in Emergency Department. Hematol. Oncol. 2013, 31, 189–196. [Google Scholar] [CrossRef]
- Smith, T.J.; Bohlke, K.; Cross, S.J.; Khatcheressian, J.L.; Lyman, G.H.; Carson, K.R.; Crawford, J.; Goldberg, J.M.; Leighl, N.B.; Perkins, C.L.; et al. Recommendations for the use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2015, 33, 3199–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aapro, M.S.; Bohlius, J.; Cameron, D.A.; Lago, L.D.; Donnelly, J.P.; Kearney, N.; Lyman, G.H.; Pettengell, R.; Tjan-Heijnen, V.; Walewski, J.; et al. 2010 Update of EORTC Guidelines for the use of Granulocyte-Colony Stimulating Factor to Reduce the Incidence of Chemotherapy-Induced Febrile Neutropenia in Adult Patients with Lymphoproliferative Disorders and Solid Tumours. Eur. J. Cancer 2011, 47, 8–32. [Google Scholar] [CrossRef]
- Crawford, J.; Becker, P.S.; Armitage, J.O.; Blayney, D.W.; Chavez, J.; Curtin, P.; Dinner, S.; Fynan, T.; Gojo, I.; Griffiths, E.A.; et al. Myeloid Growth Factors, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 1520–1541. [Google Scholar] [CrossRef] [Green Version]
- Sakurada, T.; Bando, S.; Zamami, Y.; Goda, M.; Kirino, Y.; Nakamura, T.; Teraoka, K.; Ishizawa, K.; Takechi, K.; Chuma, M.; et al. Prophylactic Administration of Granulocyte Colony-Stimulating Factor in Epirubicin and Cyclophosphamide Chemotherapy for Japanese Breast Cancer Patients: A Retrospective Study. Cancer Chemother. Pharmacol. 2019, 84, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Leonard, R.C.F.; Mansi, J.L.; Keerie, C.; Yellowlees, A.; Crawford, S.; Benstead, K.; Matthew, R.; Adamson, D.; Chan, S.; Grieve, R. A Randomised Trial of Secondary Prophylaxis using Granulocyte Colony-Stimulating Factor (‘SPROG’ Trial) for Maintaining Dose Intensity of Standard Adjuvant Chemotherapy for Breast Cancer by the Anglo-Celtic Cooperative Group and NCRN. Ann. Oncol. 2015, 26, 2437–2441. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, T.; Suzuki, K.; Sugisaki, T.; Kobayashi, K.; Shouji, D.; Watanabe, H.; Kawakami, K.; Takiguchi, T.; Aoyama, T.; Kobayashi, K. Impact of Primary Pegfilgrastim Prophylaxis on Relative Dose Intensity in Neoadjuvant/Adjuvant FEC-100 Chemotherapy. Anticancer Res. 2020, 40, 915–921. [Google Scholar] [CrossRef]
- Chan, A.; McGregor, S.; Liang, W. Utilisation of Primary and Secondary G-CSF Prophylaxis Enables Maintenance of Optimal Dose Delivery of Standard Adjuvant Chemotherapy for Early Breast Cancer: An Analysis of 1655 Patients. Breast J. 2014, 23, 676–682. [Google Scholar] [CrossRef]
- Freyer, G.; Jovenin, N.; Yazbek, G.; Villanueva, C.; Hussain, A.; Berthune, A.; Rotarski, M.; Simon, H.; Boulanger, V.; Hummelsberger, M.; et al. Granocyte-Colony Stimulating Factor (G-CSF) has Significant Efficacy as Secondary Prophylaxis of Chemotherapy-Induced Neutropenia in Patients with Solid Tumors: Results of a Prospective Study. Anticancer Res. 2013, 33, 301–308. [Google Scholar] [PubMed]
- Jones, S.; Holmes, F.A.; O’Shaughnessy, J.; Blum, J.L.; Vukelja, S.J.; McIntyre, K.J.; Pippen, J.E.; Bordelon, J.H.; Kirby, R.L.; Sandbach, J. Docetaxel with Cyclophosphamide is Associated with an overall Survival Benefit Compared with Doxorubicin and Cyclophosphamide: 7-Year Follow-Up of US Oncology Research Trial 9735. J. Clin. Oncol. 2009, 27, 1177–1183. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Lyman, G.H.; Kuderer, N.M.; Crawford, J.; Wolff, D.A.; Culakova, E.; Poniewierski, M.S.; Dale, D.C. Predicting Individual Risk of Neutropenic Complications in Patients Receiving Cancer Chemotherapy. Cancer 2011, 117, 1917–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyman, G.H.; Abella, E.; Pettengell, R. Risk Factors for Febrile Neutropenia among Patients with Cancer Receiving Chemotherapy: A Systematic Review. Crit. Rev. Oncol. 2014, 90, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, W.; Wong, M.; Malin, J. Development and Validation of a Prediction Model for the Risk of Developing Febrile Neutropenia in the First Cycle of Chemotherapy among Elderly Patients with Breast, Lung, Colorectal, and Prostate Cancer. Support Care Cancer 2011, 19, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, B.J.; Kyoung, M.K.; Bilegsaikhan, S.E.; Yong, J.S. Machine Learning Improves the Prediction of Febrile Neutropenia in Korean Inpatients Undergoing Chemotherapy for Breast Cancer. Sci. Rep. 2020, 10, 14803. [Google Scholar] [CrossRef] [PubMed]
- Morrison, V.A.; Picozzi, V.; Scott, S.; Pohlman, B.; Dickman, E.; Lee, M.; Lawless, G.; Kerr, R.; Caggiano, V.; Delgado, D.; et al. The Impact of Age on Delivered Dose Intensity and Hospitalizations for Febrile Neutropenia in Patients with Intermediate-Grade Non-Hodgkin’s Lymphoma Receiving Initial CHOP Chemotherapy: A Risk Factor Analysis. Clin. Lymphoma 2001, 2, 47–56. [Google Scholar] [CrossRef]
- Alenzi, E.O.; Kelley, G.A. The Association of Hyperglycemia and Diabetes Mellitus and the Risk of Chemotherapy-Induced Neutropenia among Cancer Patients: A Systematic Review with Meta-Analysis. J. Diabetes Complicat. 2017, 31, 267–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srokowski, T.P.; Fang, S.; Hortobagyi, G.N.; Giordano, S.H. Impact of Diabetes Mellitus on Complications and Outcomes of Adjuvant Chemotherapy in Older Patients with Breast Cancer. J. Clin. Oncol. 2009, 27, 2170–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, C.; Page, J.H.; Yang, S.; Rodriguez, R.; Huynh, J.; Chia, V.M. History of Chronic Comorbidity and Risk of Chemotherapy-Induced Febrile Neutropenia in Cancer Patients Not Receiving G-CSF Prophylaxis. Ann. Oncol. 2014, 25, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Kusano, Y.; Nishihara, A.; Inoue, N.; Nishimura, N.; Mishima, Y.; Terui, Y.; Nukada, T.; Nomura, T.; Hatake, K. Incidence and Risk Factors for Febrile Neutropenia in Japanese Patients with Non-Hodgkin B Cell Lymphoma Receiving R-CHOP: 2-Year Experience in a Single Center (STOP FN in NHL 2). Support Care Cancer 2020, 28, 571–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, E.A.; O’Mahony, M.S. Adverse Drug Reactions in Special Populations—The Elderly. Br. J. Clin. Pharmacol. 2015, 80, 796–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shayne, M.; Culakova, E.; Lyman, G.H.; Crawford, J.; Dale, D.C. Predictors of Reduced Dose Intensity in Patients with Early-Stage Breast Cancer Receiving Adjuvant Chemotherapy. Breast Cancer Res. Treat. 2006, 100, 255–262. [Google Scholar] [CrossRef]
- Damodar, G.; Smitha, T.; Gopinath, S.; Vijayakumar, S.; Rao, Y. An Evaluation of Hepatotoxicity in Breast Cancer Patients Receiving Injection Doxorubicin. Ann. Med. Health Sci. Res. 2014, 4, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Krens, S.D.; Lassche, G.; Jansman, F.G.A.; Desar, I.M.E.; Lankheet, N.A.G.; Burger, D.M.; van Herpen, C.M.L.; van Erp, N.P. Dose Recommendations for Anticancer Drugs in Patients with Renal or Hepatic Impairment. Lancet Oncol. 2019, 20, e200–e207. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Wang, T.; Xie, Y.; Li, J.; Ouyang, T.; Fan, Z. Efficacy of Pegfilgrastim to Support Neoadjuvant Dose-Dense Epirubicin and Cyclophosphamide Chemotherapy in Breast Cancer. Support Care Cancer 2019, 27, 3019–3025. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Number (%) |
|---|---|
| Age (years), median (IQR) | 50 (45–58) |
| Body mass index (kg/m2), median (IQR) | 23.8 (21.7–26.2) |
| Body surface area (m2), median (IQR) | 1.6 (1.5–1.7) |
| ER status | |
| Negative | 58 (18.1%) |
| Positive | 262 (81.9%) |
| PR status | |
| Negative | 149 (46.6%) |
| Positive | 171 (53.4%) |
| HER2 status | |
| Negative | 275 (85.9%) |
| Positive | 45 (14.1%) |
| Cancer stage | |
| I | 97 (30.3%) |
| II | 181 (56.6%) |
| III | 42 (13.1%) |
| Comorbidities | |
| Cardiovascular diseases | 54 (16.9%) |
| Diabetes mellitus | 25 (7.8%) |
| Thyroid diseases | 20 (6.3%) |
| Rheumatoid diseases | 3 (0.9%) |
| Menopause status | |
| Pre-menopause | 177 (55.3%) |
| Post-menopause | 143 (44.7%) |
| Use of G-CSF | |
| Secondary prophylaxis | 108 (33.8%) |
| Lipegfilgrastim | 63 (58.3%) |
| Pegfilgrastim | 45 (41.7%) |
| Treatment | 65 (20.3%) |
| None | 147 (45.9%) |
| Basal laboratory values, median (IQR) | |
| WBC count (×103/μL) | 6.2 (5.2–7.2) |
| ANC (cells/mm3) | 3270 (2530–4130) |
| Hemoglobin (g/dL) | 13.1 (12.3–13.7) |
| Platelet count (×103/μL) | 249.0 (218.3–295.0) |
| AST (IU/L) | 24.0 (20.0–28.0) |
| ALT (IU/L) | 17.0 (13.0–24.8) |
| Category | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Variables | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value |
| FN (N = 320) | ||||
| BSA ≥ 1.65 (vs. <1.65) | 0.55 (0.23–1.33) | 0.19 | 0.45(0.18–1.14) | 0.09 |
| Cancer stage: III over I–II | 4.03 (1.73–9.37) | <0.01 | 4.20(1.75–10.06) | <0.01 |
| DM present over absent | 3.57 (1.30–9.77) | 0.01 | 3.61(1.24–10.44) | 0.02 |
| Grade 4 Neutropenia (N = 320) | ||||
| ER: positive over negative | 1.53 (0.85–2.78) | 0.16 | 1.78(0.92–3.44) | 0.09 |
| Cancer stage: III over I–II | 8.63 (3.70–20.13) | <0.01 | 8.90(3.74–21.07) | <0.01 |
| Basal Hb < 12 g/dL | 2.46 (1.30–4.65) | 0.01 | 2.23(1.14–4.38) | 0.02 |
| Basal WBC count < 4000/μL | 3.72 (0.97–14.30) | 0.06 | 2.98(0.71–12.61) | 0.14 |
| Severe Neutropenic Events (N = 320) | ||||
| Cancer stage: III over I–II | 14.29(4.96–41.17) | <0.01 | 13.96(4.82–40.46) | <0.01 |
| Basal Hb < 12 g/dL | 2.55(1.33–4.87) | <0.01 | 2.41(1.21–4.78) | 0.01 |
| Basal WBC count < 4000/μL | 3.18 (0.83–12.20) | 0.09 | 2.51(0.59–10.70) | 0.21 |
| Variables | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Age ≥ 65 years (vs. <65) | 0.67 (0.07–6.17) | 0.72 | - | |
| BMI ≥ 25 (vs. <25) | 1.61 (0.75–3.46) | 0.22 | - | |
| BSA ≥ 1.65 (vs. <1.65) | 0.85 (0.38–1.90) | 0.70 | - | |
| ER: positive over negative | 0.82 (0.31–2.18) | 0.69 | - | |
| PR: positive over negative | 1.40 (0.65–3.03) | 0.39 | - | |
| HER2: positive over negative | 1.22 (0.46–3.27) | 0.69 | - | |
| Cancer stage: III over I–II | 1.20 (0.52–2.77) | 0.66 | - | |
| CV diseases present over absent | 2.10 (0.84–5.21) | 0.11 | 1.58 (0.58–4.29) | 0.37 |
| DM present over absent | 0.79 (0.21–3.06) | 0.74 | - | |
| Post-menopause over pre-menopause | 1.69 (0.79–3.62) | 0.17 | 2.02 (0.85–4.80) | 0.11 |
| Basal Hb < 12 g/dL | 0.78 (0.30–2.01) | 0.61 | - | |
| Basal WBC count < 4000/μL | 0.44 (0.05–3.74) | 0.45 | - | |
| Basal ALT or AST > 40 IU/L | 0.69 (0.21–2.22) | 0.53 | - | |
| Secondary prophylaxis of G-CSF | 0.20 (0.08–0.47) | <0.01 | 0.17 (0.07–0.43) | <0.01 |
| Variables | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Age ≥ 65 years (vs. <65) | 5.20 (0.80–33.68) | 0.08 | 11.78 (1.51–91.64) | 0.02 |
| BMI ≥ 25 (vs. <25) | 1.38 (0.50–3.83) | 0.54 | - | |
| BSA ≥ 1.65 (vs. <1.65) | 1.01 (0.35–2.93) | 0.98 | - | |
| ER: positive over negative | 3.59 (0.45–28.54) | 0.23 | - | |
| PR: positive over negative | 1.13 (0.40–3.17) | 0.82 | - | |
| Cancer stage: III over I–II | 1.15 (0.37–3.51) | 0.81 | - | |
| CV diseases present over absent | 2.08 (0.66–6.57) | 0.21 | - | |
| DM present over absent | 0.56 (0.07–4.62) | 0.59 | - | |
| Thyroid diseases present over absent | 0.88 (0.10–7.47) | 0.90 | - | |
| Post-menopause over pre-menopause | 1.47 (0.53–4.07) | 0.46 | - | |
| Basal Hb < 12 g/dL | 0.74 (0.20–2.76) | 0.65 | - | |
| Basal WBC count < 4000/μL | 1.19 (0.13–10.51) | 0.88 | - | |
| Basal ALT or AST > 40 IU/L | 3.15 (0.97–10.30) | 0.06 | 2.99 (0.81–11.07) | 0.10 |
| ALT or AST > 60 IU/L elevation | 3.06 (1.08–8.70) | 0.04 | 3.51 (1.09–11.37) | 0.04 |
| Recurrence of SNE | 0.54 (0.15–2.01) | 0.36 | - | |
| Secondary prophylaxis of G-CSF | 0.32 (0.11–0.94) | 0.04 | 0.27 (0.08–0.86) | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.H.; Geum, M.J.; Kang, J.E.; Park, N.G.; Oh, Y.K.; Rhie, S.J. Clinical Outcomes of Secondary Prophylactic Granulocyte Colony-Stimulating Factors in Breast Cancer Patients at a Risk of Neutropenia with Doxorubicin and Cyclophosphamide-Based Chemotherapy. Pharmaceuticals 2021, 14, 1200. https://doi.org/10.3390/ph14111200
Choi JH, Geum MJ, Kang JE, Park NG, Oh YK, Rhie SJ. Clinical Outcomes of Secondary Prophylactic Granulocyte Colony-Stimulating Factors in Breast Cancer Patients at a Risk of Neutropenia with Doxorubicin and Cyclophosphamide-Based Chemotherapy. Pharmaceuticals. 2021; 14(11):1200. https://doi.org/10.3390/ph14111200
Chicago/Turabian StyleChoi, Jae Hee, Min Jung Geum, Ji Eun Kang, Nam Gi Park, Yun Kyoung Oh, and Sandy Jeong Rhie. 2021. "Clinical Outcomes of Secondary Prophylactic Granulocyte Colony-Stimulating Factors in Breast Cancer Patients at a Risk of Neutropenia with Doxorubicin and Cyclophosphamide-Based Chemotherapy" Pharmaceuticals 14, no. 11: 1200. https://doi.org/10.3390/ph14111200
APA StyleChoi, J. H., Geum, M. J., Kang, J. E., Park, N. G., Oh, Y. K., & Rhie, S. J. (2021). Clinical Outcomes of Secondary Prophylactic Granulocyte Colony-Stimulating Factors in Breast Cancer Patients at a Risk of Neutropenia with Doxorubicin and Cyclophosphamide-Based Chemotherapy. Pharmaceuticals, 14(11), 1200. https://doi.org/10.3390/ph14111200