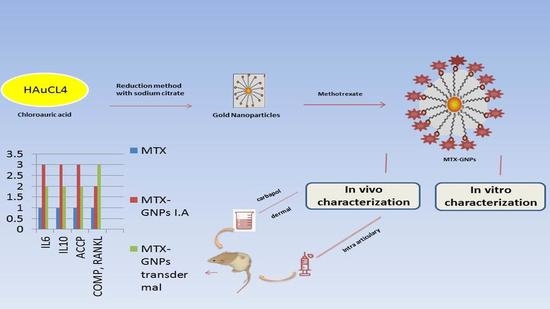
Nano Methotrexate versus Methotrexate in Targeting Rheumatoid Arthritis
Abstract
:1. Introduction
2. Results
2.1. In Vitro Evaluation of MTX-GNPs Formulation
2.2. In Vivo Evaluation of MTX-GNPs Formulation
- (a)
- Each value represents the mean of six experimental rats ± SEM. Statistical analysis was performed using one-way ANOVA followed by the Student–Newman–Keuls multiple comparisons test. a = significantly different from control group at p < 0.05; b = significantly different from MTX group at p < 0.05; c = significantly different from MTX (Nano Soln) group at p < 0.05; d = significantly different from MTX (Nano Gel) group at p < 0.05; e = significantly different from CFA group at p < 0.05; f = significantly different from CFA + MTX group at p < 0.05.
- (b)
- Each value represents the mean of six experimental rats ± SEM. Statistical analysis was performed using one-way ANOVA followed by Student–Newman–Keuls multiple comparisons test. a = significantly different from control group at p < 0.05; b = significantly different from MTX group at p < 0.05; c = significantly different from MTX (Nano Soln) group at p < 0.05; d = significantly different from MTX (Nano Gel) group at p < 0.05; e = significantly different from CFA group at p < 0.05; f = significantly different from CFA + MTX group at p < 0.05.
- (c)
- Each value represents the mean of six experimental rats ± SEM. Statistical analysis was performed using one-way ANOVA followed by Student–Newman–Keuls multiple comparisons test. a = significantly different from control group at p < 0.05; b = significantly different from MTX group at p < 0.05; c = significantly different from MTX (Nano Soln) group at p < 0.05; d = significantly different from MTX (Nano Gel) group at p < 0.05; e = significantly different from CFA group at p < 0.05; f = significantly different from CFA + MTX group at p < 0.05.
- (d)
- Each value represents the mean of six experimental rats ± SEM. Statistical analysis was performed using one-way ANOVA followed by Student–Newman–Keuls multiple comparisons test. a = significantly different from control group at p < 0.05; b = significantly different from MTX group at p < 0.05; c = significantly different from MTX (Nano Soln) group at p < 0.05; d = significantly different from MTX (Nano Gel) group at p < 0.05; e = significantly different from CFA group at p < 0.05; f = significantly different from CFA + MTX group at p < 0.05; g = significantly different from CFA + MTX (Nano Gel) group at p < 0.05.
- (e)
- Each value represents the mean of six experimental rats ± SEM. Statistical analysis was performed using one-way ANOVA followed by Student–Newman–Keuls multiple comparisons test. a = significantly different from control group at p < 0.05; b = significantly different from MTX group at p < 0.05; c = significantly different from MTX (Nano Soln) group at p < 0.05; d = significantly different from MTX (Nano Gel) group at p < 0.05; e = significantly different from CFA group at p < 0.05; f = significantly different from CFA + MTX group at p < 0.05.
- (f)
- Histopathologic examinations under a light microscope on knee joints by H&E staining (magnification ×200 and ×100).
3. Discussion
3.1. Materials
3.2. Preparation of Methotrexate-Loaded Gold Nanoparticle Formulation
| Release Data Models | Parameters of Goodness of Fit | ||
|---|---|---|---|
| R2 | MSC | AIC | |
| Zero-order | 0.7873 | 0.9968 | 47.5961 |
| First-order | 0.8398 | 1.2805 | 45.0428 |
| Higuchi | 0.9922 | 3.578 | 24.3587 |
| Korsmeyer-Peppas | 0.9844 | 3.388 | 26.0736 |
| Hixson-Crowell | 0.8209 | 1.1671 | 45.8496 |
| Hopfenberg | 0.8368 | 1.0376 | 47.0152 |
| Quadratic | 0.8974 | 1.5014 | 42.8408 |
| Baker-Lonsdale | 0.9803 | 3.39 | 25.844 |
| Formula No | X1 | X2 | X3 | Y1 | Y2 | Y3 | Y4 |
|---|---|---|---|---|---|---|---|
| F1 | 0.01 | 0.0025 | 0.0025 | 47.42 ± 2.10 | 60.99 ± 0.57 | 458.56 ± 35.42 | 0.612 |
| F2 | 0.03 | 0.0016 | 0.002 | 80.32 ± 0.42 | 34.51 ± 0.49 | 369.1 ± 3.60 | 0.549 |
| F3 | 0.02 | 0.00075 | 0.002 | 89.98 ± 1.03 | 35.83 ± 0.76 | 416.6 ± 8.83 | 0.585 |
| F4 | 0.02 | 0.0016 | 0.0025 | 64.91 ± 2.67 | 47.80 ± 0.88 | 436.21 ± 20.17 | 0.410 |
| F5 | 0.01 | 0.0016 | 0.002 | 48.31 ± 1.18 | 64.81 ± 1.02 | 381.63 ± 8.43 | 0.422 |
| F6 | 0.03 | 0.0025 | 0.0025 | 61.35 ± 2.19 | 60.53 ± 0.87 | 144.4 ± 1.76 | 0.327 |
| F7 | 0.02 | 0.0016 | 0.0025 | 64.52 ± 4.43 | 47.80 ± 0.96 | 391.2 ± 49.49 | 0.578 |
| F8 | 0.01 | 0.0016 | 0.003 | 42.53 ± 1.04 | 27.03 ± 0.89 | 241.43 ± 8.0 | 0.364 |
| F9 | 0.02 | 0.00075 | 0.003 | 89.52 ± 1.92 | 32.01 ± 0.58 | 863.23 ± 52.60 | 0.872 |
| F10 | 0.03 | 0.0016 | 0.003 | 75.89 ± 4.40 | 50.64 ± 0.64 | 320 ± 5.67 | 0.354 |
| F11 | 0.01 | 0.00075 | 0.0025 | 68.76 ± 2.15 | 32.20 ± 0.61 | 157.4 ± 19.51 | 0.356 |
| F12 | 0.02 | 0.0025 | 0.002 | 61.09 ± 4.76 | 32.62 ± 0.74 | 861.1 ± 2.61 | 0.856 |
| F13 | 0.02 | 0.0016 | 0.0025 | 63.96 ± 3.60 | 47.80 ± 0.86 | 391.2 ± 49.49 | 0.578 |
| F14 | 0.03 | 0.00075 | 0.0025 | 92.05 ± 1.74 | 33.89 ± 0.77 | 315.2 ± 14.91 | 0.446 |
| F15 | 0.02 | 0.0025 | 0.003 | 53.49 ± 0.92 | 49.97 ± 0.56 | 845.6 ± 2.45 | 0.823 |
3.3. In Vitro Evaluation of MTX-GNP Formulation
3.3.1. Measurement of % Entrapment Efficiency
3.3.2. Evaluation of Particle Size, Polydispersity Index, and Zeta Potential
3.3.3. In Vitro Release Studies
3.3.4. Kinetic Analysis of Release Data
3.4. In Vivo Characterization
3.4.1. Animals
3.4.2. Experimental Design
- Group 1: normal untreated control group, rats were just given saline and were not given any medicine.
- Group 2: CFA-arthritic group, rats were given three subcutaneous doses of CFA on days 1, 5, and 9, each dose containing 0.4 mL into the right hind legs plantar surface.
- Group 3: MTX-standard group, from day 14 to day 28, rats received MTX intra-articularly at a dose of 0.1 mg/kg/day (one dose per week).
- Group 4: MTX drug loaded on GNPs in solution form, rats received MTX-GNPs IA at a dose of 0.1 mg/kg/day from day 14 till 28 one dose per week.
- Group 5: MTX drug loaded on GNPs in gel form, 1 g MTX-GNP gel formula containing 0.1 mg active constituent applied transdermal from day 14 till 28 in a continuous manner (transdermal application of the gel every 12 h).
- Group 6: CFA-MTX drug, rats received a combination of CFA and then were treated with MTX drug (0.1 mg/kg/day) on day 14 and day 28.
- Group 7: CFA-MTX drug loaded on GNPs in solution form, rats received a combination of CFA, and then were treated with MTX-GNPs IA at a dose of 0.1 mg/kg/day from day 14 till 28, one dose per week.
- Group 8: CFA-MTX drug loaded on GNPs in gel form, rats received a combination of CFA and then were treated with 1 g MTX-GNP gel formula applied transdermal from day 14 till 28 in a continuous manner (two doses per day every 12 h). CFA dose and MTX dose have been chosen based on a previously published study [48].
3.5. Methods
3.5.1. Induction of RA
3.5.2. Blood Sampling
3.5.3. Tissue Sampling
3.5.4. Serum Levels of ACCP, IL-6, and IL-10
3.5.5. Protein Extraction and Western Blot Analysis of RANKL
3.5.6. RNA Extraction and (Q-PCR) (Real-Time Polymerase Chain Reaction)
3.5.7. Histopathological Examination
3.5.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sadarani, B.; Majumdar, A.; Paradkar, S.; Mathur, A.; Sachdev, S.; Mohanty, B.; Chaudhari, P. Enhanced skin permeation of Methotrexate from penetration enhancer containing vesicles: In vitro optimization and in vivo evaluation. Biomed. Pharmacother. 2019, 114, 108770. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Casabella, A.; Paolino, S.; Pizzorni, C.; Ghio, M.; Seriolo, C.; Molfetta, L.; Odetti, P.; Smith, V.; Cutolo, M. Dickkopf-1 (Dkk-1) serum levels in systemic sclerosis and rheumatoid arthritis patients: Correlation with the Trabecular Bone Score (TBS). Clin. Rheumatol. 2018, 37, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Guajardo-Jauregui, N.; Galarza-Delgado, D.Á.; Azpiri-López, J.R.; Colunga-Pedraza, I.J.; Cárdenas, A.; Garza-Cisneros, A.N.; Garcia-Heredia, A.; Balderas-Palacios, M.A.; Rodriguez-Romero, A.B. POS0577 Comparison of the Who and Acc/Aha Cardiovascular Algorithms to Detect Carotid Plaque in Rheumatoid Arthritis. BMJ Ann. Rheum. Dis. 2022, 81, 555. [Google Scholar] [CrossRef]
- Joshi, D.C.; Naskar, A.; Datta, K.; Sarkar, U.; Otia, M.K.; Khatoon, T. Rheumatoid Arthrities: Etiology Pathophysiology and Modern Treatments. Int. J. Res. Appl. Sci. Biotechnol. 2022, 9, 32–39. [Google Scholar] [CrossRef]
- Akiyama, M.; Kaneko, Y. Pathogenesis, clinical features, and treatment strategy for rheumatoid arthritis-associated interstitial lung disease. Autoimmun. Rev. 2022, 21, 103056. [Google Scholar] [CrossRef]
- Finckh, A.; Gilbert, B.; Hodkinson, B.; Bae, S.-C.; Thomas, R.; Deane, K.D.; Alpizar-Rodriguez, D.; Lauper, K. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 2022, 18, 591–602. [Google Scholar] [CrossRef]
- Prasad, R.; Koul, V. Transdermal delivery of methotrexate: Past, present and future prospects. Ther. Deliv. 2012, 3, 315–325. [Google Scholar] [CrossRef]
- Carretero, G.; Puig, L.; Dehesa, L.; Carrascosa, J.; Ribera, M.; Sánchez-Regaña, M.; Daudén, E.; Vidal, D.; Alsina, M.; Muñoz-Santos, C.; et al. Guidelines on the Use of Methotrexate in Psoriasis. Actas Dermo-Sifiliogr. (Engl. Ed.) 2010, 101, 600–613. [Google Scholar] [CrossRef]
- Avasatthi, V.; Pawar, H.; Dora, C.P.; Bansod, P.; Gill, M.S.; Suresh, S. A novel nanogel formulation of methotrexate for topical treatment of psoriasis: Optimization, in vitro and in vivo evaluation. Pharm. Dev. Technol. 2015, 21, 554–562. [Google Scholar] [CrossRef]
- Dehshahri, A.; Kumar, A.; Madamsetty, V.S.; Uzieliene, I.; Tavakol, S.; Azedi, F.; Fekri, H.S.; Zarrabi, A.; Mohammadinejad, R.; Thakur, V.K. New Horizons in Hydrogels for Methotrexate Delivery. Gels 2021, 7, 2. [Google Scholar] [CrossRef]
- Maudens, P.; Jordan, O.; Allémann, E. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov. Today 2018, 23, 1761–1775. [Google Scholar] [CrossRef]
- Li, W.; Cao, Z.; Liu, R.; Liu, L.; Li, H.; Li, X.; Chen, Y.; Lu, C.; Liu, Y. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4222–4233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, H.F.; Gamal, A.; Saeed, H.; Kamal, M.; Tulbah, A.S. Enhancing the Bioavailability and Efficacy of Vismodegib for the Control of Skin Cancer: In Vitro and In Vivo Studies. Pharmaceuticals 2022, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; O’Dell, J.R. State-of-the-art: Rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Shinde, C.G.; Kumar, T.M.P.; Venkatesh, M.P.; Rajesh, K.S.; Srivastava, A.; Osmani, R.A.M.; Sonawane, Y.H. Intra-articular delivery of a methotrexate loaded nanostructured lipid carrier based smart gel for effective treatment of rheumatic diseases. RSC Adv. 2016, 6, 12913–12924. [Google Scholar] [CrossRef]
- Prabhu, P.; Shetty, R.; Koland, M.; Bhat, V.; Vijayalakshmi, K.; Nairy, M.H.; Shetty, N. Investigation of nano lipid vesicles of methotrexate for anti-rheumatoid activity. Int. J. Nanomed. 2012, 7, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Fang, Z.; Yao, L.; Dahmani, F.Z.; Yin, L.; Zhou, J.; Yao, J. A micelle-like structure of poloxamer-methotrexate conjugates as nanocarrier for methotrexate delivery. Int. J. Pharm. 2015, 487, 177–186. [Google Scholar] [CrossRef]
- Salem, H.F.; Gamal, A.; Saeed, H.; Tulbah, A.S. The Impact of Improving Dermal Permeation on the Efficacy and Targeting of Liposome Nanoparticles as a Potential Treatment for Breast Cancer. Pharmaceutics 2021, 13, 1633. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tsai, C.-Y.; Huang, P.-Y.; Chang, M.-Y.; Cheng, P.-C.; Chou, C.-H.; Chen, D.-H.; Wang, C.-R.; Shiau, A.-L.; Wu, C.-L. Methotrexate Conjugated to Gold Nanoparticles Inhibits Tumor Growth in a Syngeneic Lung Tumor Model. Mol. Pharm. 2007, 4, 713–722. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ardjmand, M.; Rad, A.S.; Esfahani, M.R. Gelatin-coated gold nanoparticles as an effective pH-sensitive methotrexate drug delivery system for breast cancer treatment. Mater. Today Chem. 2021, 20, 100474. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin Loaded on Gold Nanoparticles as a Drug Delivery System for a Successful Biocompatible, Anti-Cancer, Anti-Inflammatory and Phagocytosis Inducer Model. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chuacharoen, T.; Prasongsuk, S.; Sabliov, C.M. Effect of Surfactant Concentrations on Physicochemical Properties and Functionality of Curcumin Nanoemulsions Under Conditions Relevant to Commercial Utilization. Molecules 2019, 24, 2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, F.; Diniz, L.; Sousa, R.; Honorato, T.; Simão, D.; Araújo, C.; Gonçalves, T.; Rolim, L.; Goto, P.; Tedesco, A.; et al. Preparation and characterization of nanoemulsion containing a natural naphthoquinone. Química Nova 2018, 41, 756–761. [Google Scholar] [CrossRef]
- El-Ghafar, O.A.M.A.; Helal, G.K.; Abo-Youssef, A.M. Apixaban exhibits anti-arthritic effects by inhibiting activated factor X-mediated JAK2/STAT3 and MAPK phosphorylation pathways. Inflammopharmacology 2020, 28, 1253–1267. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; He, X.; Li, Z.; Shi, B.; Cai, F. β-Sitosterol-loaded solid lipid nanoparticles ameliorate complete Freund’s adjuvant-induced arthritis in rats: Involvement of NF-кB and HO-1/Nrf-2 pathway. Drug Deliv. 2020, 27, 1329–1341. [Google Scholar] [CrossRef]
- Parnsamut, C.; Brimson, S. Effects of silver nanoparticles and gold nanoparticles on IL-2, IL-6, and TNF-α production via MAPK pathway in leukemic cell lines. Genet. Mol. Res. 2015, 14, 3650–3668. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.M.; Saleh, H. Promising therapeutic effect of gold nanoparticles against dinitrobenzene sulfonic acid-induced colitis in rats. Nanomedicine 2018, 13, 1657–1679. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Dai, Z.-H.; Liu, P.-C.; Chuu, J.-J.; Lee, K.-Y.; Lee, S.-L.; Chen, Y.-J. Effects of Nanogold on the Alleviation of Carbon Tetrachloride-Induced Hepatic Injury in Rats. Chin. J. Physiol. 2012, 55, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Bessar, H.; Venditti, I.; Benassi, L.; Vaschieri, C.; Azzoni, P.; Pellacani, G.; Magnoni, C.; Botti, E.; Casagrande, V.; Federici, M.; et al. Functionalized gold nanoparticles for topical delivery of methotrexate for the possible treatment of psoriasis. Colloids Surf. B Biointerfaces 2016, 141, 141–147. [Google Scholar] [CrossRef] [Green Version]
- McCullough, J.; Snyder, D.; Weinstein, G.; Friedland, A.; Stein, B. Factors Affecting Human Percutaneous Penetration of Methotrexate and Its Analogues In Vitro. J. Investig. Dermatol. 1976, 66, 103–107. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, Z.Q.; Zhu, S.F.; Gao, Y. Comparative study of methotrexate and human umbilical cord mesenchymal stem cell transplantation in the treatment of rheumatoid arthritis. J. Biol. Regul. Homeost. Agents 2018, 32, 599–605. [Google Scholar]
- Gul, A.; Kunwar, B.; Mazhar, M.; Faizi, S.; Ahmed, D.; Shah, M.R.; Simjee, S.U. Rutin and rutin-conjugated gold nanoparticles ameliorate collagen-induced arthritis in rats through inhibition of NF-κB and iNOS activation. Int. Immunopharmacol. 2018, 59, 310–317. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, T.G.; Garcia, V.B.; de Araújo, A.A.; Gasparotto, L.H.D.S.; Silva, H.; Guerra, G.C.B.; Miguel, E.D.C.; Leitão, R.F.D.C.; Costa, D.V.D.S.; Cruz, L.J.; et al. Spherical neutral gold nanoparticles improve anti-inflammatory response, oxidative stress and fibrosis in alcohol-methamphetamine-induced liver injury in rats. Int. J. Pharm. 2018, 548, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.S.; Kim, W.J.; Kim, J.J.; Kim, T.J.; Ye, S.K.; Song, M.D.; Kang, H.; Kim, D.W.; Moon, W.K.; Lee, K.H. Gold nanoparticles attenuate LPS-induced NO production through the inhibition of NF-κB and IFN-β/STAT1 pathways in RAW264.7 cells. Nitric Oxide 2010, 23, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Han, X.; Pan, S.; Wu, Y.; Jiang, Y.; Lin, J.; Chen, Y.; Jin, H. Gold Nanomaterials and Bone/Cartilage Tissue Engineering: Biomedical Applications and Molecular Mechanisms. Front. Chem. 2021, 9, 724188. [Google Scholar] [CrossRef]
- Georgiev, T.; Ivanova, M.; Kopchev, A.; Velikova, T.; Miloshov, A.; Kurteva, E.; Yuzeir, K.; Penkov, M.; Kabakchieva, P.; Rashkov, R.; et al. Cartilage oligomeric protein, matrix metalloproteinase-3, and Coll2-1 as serum biomarkers in knee osteoarthritis: A cross-sectional study. Rheumatol. Int. 2017, 38, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Yan, J.; Levy, S.; Bhagchandani, S.; Slaughter, K.V.; Sherman, N.E.; Amirault, J.; Wang, Y.; Riegel, L.; He, X.; et al. Towards an arthritis flare-responsive drug delivery system. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Ha, Y.-J.; Lee, S.-M.; Mun, C.H.; Kim, H.J.; Bae, Y.; Lim, J.-H.; Park, K.-H.; Lee, S.-K.; Yoo, K.-H.; Park, Y.-B. Methotrexate-loaded multifunctional nanoparticles with near-infrared irradiation for the treatment of rheumatoid arthritis. Thromb. Haemost. 2020, 22, 1–13. [Google Scholar] [CrossRef]
- Goldie, I.; Nachemson, A. Synovial pH in Rheumatoid Knee Joints: II. The Effect of Local Corticosteroid Treatment. Acta Orthop. 1970, 41, 354–362. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Zou, X.; Su, H.; Li, C. Methotrexate-loaded folic acid of solid-phase synthesis conjugated gold nanoparticles targeted treatment for rheumatoid arthritis. Eur. J. Pharm. Sci. 2022, 170, 106101. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wang, C.-H.; Chang, W.-R.; Li, J.-W.; Hsu, M.-F.; Sun, Y.-S.; Liu, T.-Y.; Chiu, C.-W. Hydrophilic-Hydrophobic Nanohybrids of AuNP-Immobilized Two-Dimensional Nanomica Platelets as Flexible Substrates for High-Efficiency and High-Selectivity Surface-Enhanced Raman Scattering Microbe Detection. ACS Appl. Bio Mater. 2022, 5, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Varela-Aramburu, S.; Ghosh, C.; Goerdeler, F.; Priegue, P.; Moscovitz, O.; Seeberger, P.H. Targeting and Inhibiting Plasmodium falciparum Using Ultra-small Gold Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 43380–43387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jia, Y.; Li, J.; Dong, R.; Zhang, J.; Ma, C.; Wang, H.; Rui, Y.-K.; Jiang, X. Indole Derivative-Capped Gold Nanoparticles as an Effective Bactericide In Vivo. ACS Appl. Mater. Interfaces 2018, 10, 29398–29406. [Google Scholar] [CrossRef] [PubMed]
- Ghiulai, R.; Mioc, A.; Racoviceanu, R.; Mioc, M.; Milan, A.; Prodea, A.; Semenescu, A.; Dehelean, C.; Tudoran, L.B.; Avram, Ș.; et al. The Anti-Melanoma Effect of Betulinic Acid Functionalized Gold Nanoparticles: A Mechanistic In Vitro Approach. Pharmaceuticals 2022, 15, 1362. [Google Scholar] [CrossRef] [PubMed]
- El-Ela, F.I.A.; Hussein, K.H.; El-Banna, H.A.; Gamal, A.; Rouby, S.; Menshawy, A.M.S.; El-Nahass, E.-S.; Anwar, S.; Zeinhom, M.M.A.; Salem, H.F.; et al. Treatment of Brucellosis in Guinea Pigs via a Combination of Engineered Novel pH-Responsive Curcumin Niosome Hydrogel and Doxycycline-Loaded Chitosan-Sodium Alginate Nanoparticles: An In Vitro and In Vivo Study. AAPS PharmSciTech 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Tulbah, A.S.; Gamal, A. Design and Characterization of Atorvastatin Dry Powder Formulation as a Potential Lung Cancer Treatment. Saudi Pharm. J. 2021, 29, 1449–1457. [Google Scholar] [CrossRef]
- Tulbah, A.; Pisano, E.; Scalia, S.; Young, P.M.; Traini, D.; Ong, H.X. Inhaled simvastatin nanoparticles for inflammatory lung disease. Nanomedicine 2017, 12, 2471–2485. [Google Scholar] [CrossRef]
- El-Gaphar, O.A.M.A.; Abo-Youssef, A.M.; Abo-Saif, A.A. Effect of losartan in complete freund’s adjuvant-induced arthritis in rats. Iran. J. Pharm. Res. IJPR 2018, 17, 1420. [Google Scholar]
- Ismail, C.A.N.; Noh, A.S.M.; Tan, D.C.; Khir, N.A.M.; Shafin, N. A Review on Complete Freund’s Adjuvant-Induced Arthritic Rat Model: Factors Leading to its Success. IIUM Med. J. Malays. 2022, 21, 3–12. [Google Scholar] [CrossRef]
- Gonca, G.; Sahin, B.; Parlak, A.; Ust, Y.; Ayhan, V.; Cesur, I.; Boru, B. Theoretical and experimental investigation of the Miller cycle diesel engine in terms of performance and emission parameters. Appl. Energy 2015, 138, 11–20. [Google Scholar] [CrossRef]
- Cheng, Y.; Wei, H.; Sun, R.; Tian, Z.; Zheng, X. Rapid method for protein quantitation by Bradford assay after elimination of the interference of polysorbate 80. Anal. Biochem. 2015, 494, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Chien, S.; Barakat, A.I.; Nerem, R.M. Endothelial cellular response to altered shear stress. Am. J. Physiol. Cell. Mol. Physiol. 2001, 281, L529–L533. [Google Scholar] [CrossRef] [PubMed]
- Seevaratnam, R.; Patel, B.P.; Hamadeh, M.J. Comparison of Total Protein Concentration in Skeletal Muscle as Measured by the Bradford and Lowry Assays. J. Biochem. 2009, 145, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A. A note on call-out culture. Briarpatch Magazine, 2 March 2015; 1–2. [Google Scholar]





| Gene Symbol | Primer Sequence from 5′–3′ F: Forward Primer, R: Reverse Primer | Gene Bank Accession Number |
|---|---|---|
| COMP | F: ACACAGGGTCAAGGAGATCAC | NM_012834.2 |
| R: AGACTACGCCAGGGAAGCA | ||
| β-actin | F: TCCGTCGCCGGTCCACACCC | NM_031144.3 |
| R: TCACCAACTGGGACGATATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, H.F.; Abd El-Maboud, M.M.; Said, A.S.A.; Salem, M.N.; Sabry, D.; Hussain, N.; El-Ghafar, O.A.M.A.; Hussein, R.R.S. Nano Methotrexate versus Methotrexate in Targeting Rheumatoid Arthritis. Pharmaceuticals 2023, 16, 60. https://doi.org/10.3390/ph16010060
Salem HF, Abd El-Maboud MM, Said ASA, Salem MN, Sabry D, Hussain N, El-Ghafar OAMA, Hussein RRS. Nano Methotrexate versus Methotrexate in Targeting Rheumatoid Arthritis. Pharmaceuticals. 2023; 16(1):60. https://doi.org/10.3390/ph16010060
Chicago/Turabian StyleSalem, Heba F., Marwa Mohamed Abd El-Maboud, Amira S. A. Said, Mohamed Nabil Salem, Dina Sabry, Nadia Hussain, Omnia A. M. Abd El-Ghafar, and Raghda R. S. Hussein. 2023. "Nano Methotrexate versus Methotrexate in Targeting Rheumatoid Arthritis" Pharmaceuticals 16, no. 1: 60. https://doi.org/10.3390/ph16010060
APA StyleSalem, H. F., Abd El-Maboud, M. M., Said, A. S. A., Salem, M. N., Sabry, D., Hussain, N., El-Ghafar, O. A. M. A., & Hussein, R. R. S. (2023). Nano Methotrexate versus Methotrexate in Targeting Rheumatoid Arthritis. Pharmaceuticals, 16(1), 60. https://doi.org/10.3390/ph16010060