Efficacy, Tolerability, and Safety of Toludesvenlafaxine for the Treatment of Major Depressive Disorder—A Narrative Review
Abstract
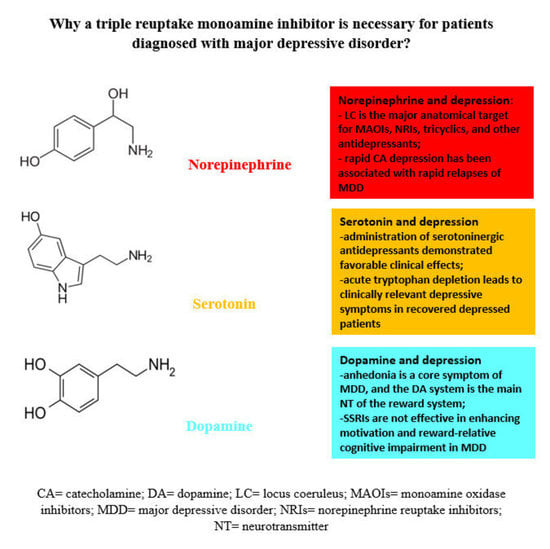
:1. Introduction
2. Preclinical and Clinical Data on the Efficacy, Tolerability, Safety, and Pharmacological Profile of Toludesvenlafaxine
2.1. Preclinical Studies
| Design | Results | Observations | Reference |
|---|---|---|---|
| Single and 13-week repeated-dose oral toxicity assessment and mutagenicity assays. Acute dose: 500 mg/kg, 1000 mg/kg, and 2000 mg/kg LPM570065 in SD rats. A 13-week toxicity study: 30 mg/kg, 100 mg/kg, or 300 mg/kg LPM570065 for 13 consecutive weeks + 4-week recovery period. N = 80 rats (40 males and 40 females). | In a single-dose acute study: 2 out of 20 rats died in the 1000 mg/kg group vs. seven out of 20 in the 2000 mg/kg group vs. none in the 500 mg/kg group. In the 13-week toxicity study: transitory salivation and minor body weight decrease was reported in the 300 mg/kg group in males. Serum PRL levels ↓ by 43% and 78% in male rats in 100 mg/kg and 300 mg/kg groups, respectively. Serum TST ↑ by 37% in the 30 mg/kg and 100 mg/kg males. | MTD = 500 mg/kg and lethal dose = 1000 mg/kg in the acute administration. In the long-term administration, no observed AE level was ≥300 mg/kg for rats; no mutagenic or clastogenic effects. MTD = 3000 mg/patient/day in clinical conditions. The effects of LPM570065 on sexual function are to be monitored. | Li C, Jiang W, Gao Y, et al. [77] |
| Acute phase: 30 mg/kg, 100 mg/kg, and 300 mg/kg LPM570065 vs. control. Female rats received 2 weeks of the investigational product + mating up to the 7th gestation day. Male rats received 4 weeks of investigational product + mating with treated female rats. Following this stage, all males were treated up to the ninth week and a new mating period was initiated with non-treated female rats. Mortality, toxicity symptoms, body weight, amount of food consumed, sexual cycle, mating behavior, pregnancy, sperm production, gross necropsy, and weight of organs. N = 264 rats were distributed in 4 groups (44 females and 22 males in each group). | Excessive salivation post-treatment in all females and males on 100 mg/kg and 300 mg/kg LPM570065 groups. BW gain ↓ in gravid rats with 300 mg/kg investigational product during gestation days 0–6. Decreased fertility rates were associated with a 300 mg/kg dose of investigational product in male rates. Sperm concentration and count were higher in all three groups treated with LPM570065 vs. controls. Duration of mating ↓ significantly to 37.5% after 9 weeks of treatment with 300 mg/kg. | The no observable AE level was established at 100 mg/kg (female rats) and 300 mg/kg (male rats). The no observable AE level for fertility and early embryonic development was established at 300 mg/kg (female rats) and 100 mg/kg (male rats). | Guo W, Gao Y, Jiang W, et al. [78] |
| Exploring extracellular 5HT, NE, and DA levels in the rat striatum after acute and chronic administration of LPM570065 vs. DSVLFX. The methods used were HPLC and microdialysis. N = 72 rats divided into 9 equal groups. | HPLC results showed that LPM570065 rapidly penetrates the striatum and converts into DSVLFX while presenting larger total exposure vs. DSVLFX. Long-term administration of LPM570065 (up to 14 days) via the oral route increased all three monoamine levels more than DSVLFX, and especially dopamine levels (detected by microdialysis). During the forced swim test, acute and chronic administration of LPM570065 ↓ the immobility time more than DSVLFX. | LPM570065 may possess an ↑ efficacy and/or a more rapid onset of antidepressant effect than DSVLFX. LPM570065 counterbalances the negative effects of DSVLFX on 5HT neurotransmission related to the 5HT1A autoreceptors. | Zhang R, Li X, Shi Y, et al. [79] |
| Adult male and female C57BL/6J mice, 5 groups, each group had 24 animals: control vs. single-stress vs. double-stress vs. LPM570065 vs. fluoxetine groups. Sucrose preference test, forced swimming test, and tail suspension test. | LPM570065 reduced susceptibility to depression-like behaviors in adult mice + maternal separation. LPM570065 protected against the reduced number of dendritic spines in the hippocampal CA1 of mice subjected to stress. LPM570065 regulated the expression of DNMTs in the mouse hippocampus. | LPM570065 may reduce depression vulnerability via epigenetic mechanisms involving the Oxtr expression. | Meng P, Li C, Duan S, et al. [80] |
| Male and female Wistar and Sprague–Dawley rats (total of 12/sex/group and 5/sex/group, respectively); affinity for monoamine transporters was determined by radioligand membrane binding assay; chronic unpredictable mild stress procedure; rat olfactory bulbectomized model, open field test, sucrose consumption test, serum corticosterone, and testosterone levels. Toludesvenlafaxine 10 μM. | The highest inhibition for serotonin transporters was reported in in vitro assays. The absorption was good after oral administration, and it was converted to O-desvenlafaxine due to the action of esterases in vivo, both reaching the hypothalamus in high concentration. | While desvenlafaxine does not increase the striatal level of dopamine, toludesvenlafaxine has this effect, which indicates supplementary benefits vs. the older drug. | Zhu H, Wang W, Sha C, et al. [81] |
2.2. Clinical Trials
| Methodology | Primary Outcome(s) and Measures | Secondary Outcome(s) and Measures | Sponsor of the Clinical Trial | The Country Where the Clinical Trial Took Place | Status of the Trial | Results and Observations | Registration of Clinical Trial and/or Reference(s) |
|---|---|---|---|---|---|---|---|
| LY03005 (40 mg, 80 mg, 120 mg, and 160 mg) vs. placebo, DBRCT, phase 2, dose-finding study, N = 260 MDD patients (18–65 years old), 2 weeks wash out + 6 weeks treatment | HAMD-17 scores at week 8 | MADRS and CGI-I at week 8 | Luye Pharma Group Ltd. (China) | China | Completed | HAMD-17 scores were significantly changed by the intervention vs. placebo at week 6 in all active treatment groups vs. placebo (p < 0.05). All doses were generally well tolerated, but the % of AEs was superior to the placebo group in each active treatment group. | NCT03785652 [62,91] |
| LY03005 (80 mg or 160 mg) vs. placebo, DBRCT, phase 3, N = 558 MDD patients (18–65 years old), 1-week screening + 8-week double-blind treatment | MADRS scores at 8 weeks | HAMD-17 at week 8 | Luye Pharma Group Ltd. (China) | China | Completed | HAMD-17 total score and “anxiety/somatization”, “cognitive impairment”, and “blocking” factors, CGI, HAM-A, SDS, and MADRS “anhedonia factor” scores were significantly improved vs. placebo at week 8. Most of the adverse events were mild and moderate, and no SAE was reported. Nausea, vomiting, headache, and drowsiness were the most frequently reported (over 5%) AEs in the active treatment groups. | NCT04853407 [82,92] |
| LY03005 (20 mg, 40 mg, 80 mg, 120 mg, 160 mg, 200 mg, and 120 mg + fed) vs. DSVLFX (50 mg) vs. placebo, phase 1, RDBCT, N = 72 healthy participants in the SAD study + 12 subjects in food effect study (18–45 years old) | Number of participants with AEs during 11 days | PK parameters-Cmax up to 4 days | Luye Pharma Group Ltd. (China) | United States | Completed | Unpublished results. No obvious effect of food on the bioavailability of LY03005. Good safety profile and linear dose proportionality on the plasma exposure after single oral dose administration. | NCT02055300 [84,86] |
| LY03005 (40 mg, 80 mg, 120 mg, or 160 mg) vs. placebo, phase 1, RDBCT, MAD, N = 48 healthy subjects (18–45 years old), 8 consecutive days | Number of participants with AEs during 3 to 4 months | The PK of MAD | Luye Pharma Group Ltd. (China) | United States | Completed | Unpublished results. Good safety profile of LY03005 treatment and linear dose proportionality on the plasma exposure after multiple oral administrations. The steady state of plasma exposure was reached after 3rd or 4th oral intake of the investigational product. | NCT02271412 [83,86] |
| Phase 1 trials, healthy volunteers (N = 132), single or multiple doses of oral LY03005 administration; N1 = 72 subjects in SAD study, N2 = 12 subjects in the food-effect study, and N3 = 48 subjects in the MAD study. | Safety and PK profiles for LY03005 | Luye Pharma Group Ltd. (China) | China | Completed | Unpublished results. SAD study: concentrations of the main active metabolite were dose proportional for the dose range of 20–200 mg LY03005. Food-effect study: food did not affect the bioavailability in healthy subjects. MAD study: the steady state of the main active metabolite could be achieved on the 3rd day following multiple dosing; concentrations of the main active metabolite were dose proportional at the steady state for the dose range 40–160 mg/day LY03005. All studies: good tolerability and safety. | CTR20130364, CTR20140333, and CTR20140418 [85] | |
| LY03005 (80 mg) vs. DSVLFX (50 mg), phase 1, pilot study, open-label study, single dose, N = 20 healthy subjects (18–50 years old) | Bioavailability of oral tablets under fasting conditions: AUC-PK samples were drawn at t0 (i.e., 30 min prior to dosing), 1 h, 2 h, 3 h, 4 h, 6 h, 8 h, 10 h, 12 h, 23 h, 32 h, 48 h, and 72 h after dosing | Luye Pharma Group Ltd. (China) | United States | Completed | Undisclosed | NCT02988024 [87] | |
| LY03005 (80 mg) fasted vs. fed crossover open-label randomized trial, single dose, phase 1, N = 34 participants (18–50 years old) | AUC and Cmax:PK parameters (predose and after dose) of parent and active metabolite | Luye Pharma Group Ltd. (China) | United States | Completed | Undisclosed | NCT03822065 [88] | |
| LY03005 (80 mg) vs. DSVLFX (50 mg) 2-sequence, 2-period crossover open-label randomized trial, phase 1, single dose, N = 56 healthy participants (18–50 years) | AUC 15 days, parent drug and its active metabolite | Luye Pharma Group Ltd. (China) | United States | Completed | Undisclosed | NCT03733574 [89] | |
| LY03005 (80 mg) vs. DSVLFX (50 mg), phase 1, randomized, open-label, cross-over, 2-period study, single dose, N = 20 healthy participants (18–50 years old) | AUC assessment up to 72 h after dosing in both trial periods. Cmax assessment up to 72 h after dosing in both trial periods. | AEs assessment up to 35 days | Luye Pharma Group Ltd. (China) | United States | Completed | Undisclosed | NCT03357796 [90] |
| Phase 1 trial, healthy volunteers | Safety and tolerability of LY03005 | Luye Pharma Group Ltd. (China) | Japan | Completed | Undisclosed | [64] |
3. Discussion
| Pharmacological Agent | SERT | NET | DAT | Observations |
|---|---|---|---|---|
| Toludesvenlafaxine | IC50 = 31.4 ± 0.4 nM | IC50 = 586.7 ± 83.6 nM | IC50 = 733.2 ± 10.3 nM | Prodrug of desvenlafaxine, TRI |
| Desvenlafaxine | IC50 = 47.3 ± 19.4 nM | IC50 = 531.3 ± 113 nM | - | Major metabolite of venlafaxine, SNRI |
| Venlafaxine | IC50 = 145 nM | IC50 = 2483 nM | IC50 = 7647 nM | SNRI |
| Duloxetine | IC50 = 13 nM | IC50 = 42 nM | IC50 = 439 nM | SNRI |
| Milnacipran | IC50 = 151 nM | IC50 = 200 nM | IC50 > 100,000 | SNRI |
| DOV 216,303 | IC50 = 14 nM | IC50 = 20 nM | IC50 = 78 nM | TRI |
| DOV 21,947 | IC50 = 12 nM | IC50 = 23 nM | IC50 = 96 nM | (+)-DOV-216,303, TRI |
| Ro 8-4650 | IC50 = 4.8 μM | IC50 = 2.5 μM | IC50 = 4.5 μM | TRI |
| SKF83959 | Ki = 1.43 ± 0.45 μmol/L | Ki = 0.6 ± 0.07 μmol/L | Ki = 9.01 ± 0.8 μmol/L | TRI |
| BMS-820836 | IC50 = 0.2 | IC50 = 26.7 nM | IC50 = 6.19 nM | TRI |
| Amitriptyline | IC50 = 67 nM | IC50 = 63 nM | IC50 = 7500 nM | Tricyclic antidepressant |
4. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Mukai, Y.; Furtek, C.I.; Bortnichak, E.A.; Liaw, K.-L.; Zhong, W. Epidemiology of Treatment-Resistant Depression in the United States. J. Clin. Psychiatry 2021, 83, 38389. [Google Scholar] [CrossRef]
- Si, T.; Wang, P. When is antidepressant polypharmacy appropriate in the treatment of depression? Shanghai Arch. Psychiatry 2014, 26, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, O.; Vasile, D. Risk factors and quality of life in late-life depressive disorders. Rom. J. Mil. Med. 2016, 119, 24–28. [Google Scholar] [CrossRef]
- Wiersema, C.; Voshaar, R.C.O.; Brink, R.H.S.V.D.; Wouters, H.; Verhaak, P.; Comijs, H.C.; Jeuring, H.W. Determinants and consequences of polypharmacy in patients with a depressive disorder in later life. Acta Psychiatr. Scand. 2022, 146, 85–97. [Google Scholar] [CrossRef]
- Vasiliu, O. Effects of the selective serotonin reuptake inhibitors over coagulation in patients with depressive dis-orders—A systematic review and retrospective analysis. RJMM 2019, 122, 7–11. [Google Scholar] [CrossRef]
- Rhee, T.G.; Rosenheck, R.A. Psychotropic polypharmacy reconsidered: Between-class polypharmacy in the context of multimorbidity in the treatment of depressive disorders. J. Affect. Disord. 2019, 252, 450–457. [Google Scholar] [CrossRef]
- Vasile, D.; Vasiliu, O.; Vasile, M.L.; Terpan, M.; Ojog, D.G. P.2.c.002 Agomelatine versus selective serotoninergic reuptake inhibitors in major depressive disorder and comorbid diabetes mellitus. Eur. Neuropsychopharmacol. 2011, 21, S383–S384. [Google Scholar] [CrossRef]
- Paulzen, M.; Haen, E.; Hiemke, C.; Fay, B.; Unholzer, S.; Gründer, G.; Schoretsanitis, G. Antidepressant polypharmacy and the potential of pharmacokinetic interactions: Doxepin but not mirtazapine causes clinically relevant changes in venlafaxine metabolism. J. Affect. Disord. 2018, 227, 506–511. [Google Scholar] [CrossRef]
- Wolff, J.; Hefner, G.; Normann, C.; Kaier, K.; Binder, H.; Hiemke, C.; Toto, S.; Domschke, K.; Marschollek, M.; Klimke, A. Polypharmacy and the risk of drug–drug interactions and potentially inappropriate medications in hospital psychiatry. Pharmacoepidemiol. Drug Saf. 2021, 30, 1258–1268. [Google Scholar] [CrossRef]
- Fond, G. A comparative analysis of effectiveness, tolerance and cost of second generation antidepressants in France. La Tunis. Med. 2015, 93, 123–128. [Google Scholar] [PubMed]
- Delgado, P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry 2000, 61 (Suppl. S6), 7–11. [Google Scholar] [PubMed]
- Heninger, G.R.; Delgado, P.L.; Charney, D.S. The Revised Monoamine Theory of Depression: A Modulatory Role for Monoamines, Based on New Findings From Monoamine Depletion Experiments in Humans. Pharmacopsychiatry 1996, 29, 2–11. [Google Scholar] [CrossRef]
- Charney, D.S. Monoamine dysfunction and the pathophysiology and treatment of depression. J. Clin. Psychiatry 1998, 59, 11–14. [Google Scholar]
- Smith, K.; Fairburn, C.; Cowen, P. Relapse of depression after rapid depletion of tryptophan. Lancet 1997, 349, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wichers, M.C.; Koek, G.H.; Robaeys, G.; Verkerk, R.; Scharpé, S.; Maes, M. IDO and interferon-α-induced depressive symptoms: A shift in hypothesis from tryptophan depletion to neurotoxicity. Mol. Psychiatry 2004, 10, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Köhler, S.; Cierpinsky, K.; Kronenberg, G.; Adli, M. The serotonergic system in the neurobiology of depression: Relevance for novel antidepressants. J. Psychopharmacol. 2016, 30, 13–22. [Google Scholar] [CrossRef]
- Nutt, D. Relationship of neurotransmitters to the symptoms of major depressive disorder. J. Clin. Psychiatry 2008, 69 (Suppl. E1), 4–7. [Google Scholar]
- Nutt, D.J. The role of dopamine and norepinephrine in depression and antidepressant treatment. J. Clin. Psychiatry 2006, 67 (Suppl. S6), 3–8. [Google Scholar] [PubMed]
- Chandley, M.J.; Ordway, G.A. Noradrenergic dysfunction in depression and suicide. In The Neurobiological Basis of Suicide; Dwivedi, Y., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK107205/#top (accessed on 23 February 2023).
- Moret, C.; Briley, M. The importance of norepinephrine in depression. Neuropsychiatr. Dis. Treat. 2011, 7 (Suppl. S1), 9–13. [Google Scholar] [CrossRef]
- Dailly, E.; Chenu, F.; Renard, C.E.; Bourin, M. Dopamine, depression and antidepressants. Fundam. Clin. Pharmacol. 2004, 18, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.; Nemeroff, C.B. The Role of Dopamine in the Pathophysiology of Depression. Arch. Gen. Psychiatry 2007, 64, 327–337. [Google Scholar] [CrossRef]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutt, D.J.; Demyttenaere, K.; Janka, Z.; Aarre, T.; Bourin, M.; Canonico, P.L.; Carrasco, J.L.; Stahl, S. The other face of depression, reduced positive affect: The role of catecholamines in causation and cure. J. Psychopharmacol. 2006, 21, 461–471. [Google Scholar] [CrossRef]
- Budisteanu, M.; Andrei, E.; Linca, F.; Hulea, D.S.; Velicu, A.C.; Mihailescu, I.; Riga, S.; Arghir, A.; Papuc, S.M.; Sirbu, C.A.; et al. Predictive factors in early onset schizophrenia. Exp. Ther. Med. 2020, 20, 210. [Google Scholar] [CrossRef] [PubMed]
- Peitl, V.; Štefanović, M.; Karlović, D. Depressive symptoms in schizophrenia and dopamine and serotonin gene polymorphisms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 77, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, R.M. History and evolution of the monoamine hypothesis of depression. J. Clin. Psychiatry 2000, 61 (Suppl. S6), 4–6. [Google Scholar]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2017, 72, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Pigott, H.E. The STAR*D Trial: It is Time to Reexamine the Clinical Beliefs That Guide the Treatment of Major Depression. Can. J. Psychiatry 2015, 60, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Vasiliu, O. Investigational Drugs for the Treatment of Depression (Part 1): Monoaminergic, Orexinergic, GABA-Ergic, and Anti-Inflammatory Agents. Front. Pharmacol. 2022, 13, 884143. [Google Scholar] [CrossRef]
- Khoodoruth, M.A.S.; Estudillo-Guerra, M.A.; Pacheco-Barrios, K.; Nyundo, A.; Chapa-Koloffon, G.; Ouanes, S. Glutamatergic System in Depression and Its Role in Neuromodulatory Techniques Optimization. Front. Psychiatry 2022, 13, 886918. [Google Scholar] [CrossRef]
- Vasiliu, O. Investigational Drugs for the Treatment of Depression (Part 2): Glutamatergic, Cholinergic, Sestrin Modulators, and Other Agents. Front. Pharmacol. 2022, 13, 884155. [Google Scholar] [CrossRef]
- Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Szabó, A.; Vécsei, L. Integrating Armchair, Bench, and Bedside Research for Behavioral Neurology and Neuropsychiatry: Editorial. Biomedicines 2022, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Pinhasov, A.; Ornoy, A. Animal Models of Depression: What Can They Teach Us about the Human Disease? Diagnostics 2021, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Slaney, C.; Hinchcliffe, J.K.; Robinson, E.S.J. Translational Shifts in Preclinical Models of Depression: Implications for Biomarkers for Improved Treatments. Curr. Top. Behav. Neurosci. 2018, 40, 169–193. [Google Scholar] [CrossRef]
- von Mücke-Heim, I.-A.; Urbina-Treviño, L.; Bordes, J.; Ries, C.; Schmidt, M.V.; Deussing, J.M. Introducing a depression-like syndrome for translational neuropsychiatry: A plea for taxonomical validity and improved comparability between humans and mice. Mol. Psychiatry 2022, 28, 329–340. [Google Scholar] [CrossRef]
- Petković, A.; Chaudhury, D. Encore: Behavioural animal models of stress, depression and mood disorders. Front. Behav. Neurosci. 2022, 16, 931964. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential Role of BDNF in the Mesolimbic Dopamine Pathway in Social Defeat Stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef] [Green Version]
- Jayatissa, M.N.; Bisgaard, C.; Tingström, A.; Papp, M.; Wiborg, O. Hippocampal Cytogenesis Correlates to Escitalopram-Mediated Recovery in a Chronic Mild Stress Rat Model of Depression. Neuropsychopharmacology 2006, 31, 2395–2404. [Google Scholar] [CrossRef] [Green Version]
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr. Dis. Treat. 2020, 16, 221–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, H.; Santra, S.; Dutta, A. Triple reuptake inhibitors as potential next-generation antidepressants: A new hope? Futur. Med. Chem. 2015, 7, 2385–2406. [Google Scholar] [CrossRef] [Green Version]
- Marks, D.M.; Pae, C.-U.; Patkar, A.A. Triple Reuptake Inhibitors: The Next Generation of Antidepressants. Curr. Neuropharmacol. 2008, 6, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Skolnick, P.; Krieter, P.; Tizzano, J.; Basile, A.; Popik, P.; Czobor, P.; Lippa, A. Preclinical and Clinical Pharmacology of DOV 216,303, a “Triple” Reuptake Inhibitor. CNS Drug Rev. 2006, 12, 123–134. [Google Scholar] [CrossRef]
- Tran, P.; Skolnick, P.; Czobor, P.; Huang, N.; Bradshaw, M.; McKinney, A.; Fava, M. Efficacy and tolerability of the novel triple reuptake inhibitor amitifadine in the treatment of patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. J. Psychiatr. Res. 2012, 46, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Euthymics Bioscience Inc. Euthymics Reports Top-Line Results from TRIADE Trial of Amitifadine for Major Depressive Disorder. Available online: https://web.archive.org/web/20170924095823/http://euthymics.com/wp-content/uploads/2013/05/FINAL_Euthymics_TRIADE_Results_052913.pdf (accessed on 27 December 2022).
- Luethi, D.; Hoener, M.; Liechti, M.E. Effects of the new psychoactive substances diclofensine, diphenidine, and methoxphenidine on monoaminergic systems. Eur. J. Pharmacol. 2018, 819, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Cherpillod, C.; Omer, L.M.O. A Controlled Trial with Diclofensine, a New Psychoactive Drug, in the Treatment of Depression. J. Int. Med. Res. 1981, 9, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Guo, L.; Jia, J.; Jin, G.-Z.; Zhao, B.; Zheng, Y.-Y.; Li, J.-Q.; Zhang, A.; Zhen, X.-C. SKF83959 is a novel triple reuptake inhibitor that elicits anti-depressant activity. Acta Pharmacol. Sin. 2013, 34, 1149–1155. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Wang, F.; Yang, S.; Fang, P.; Deng, Z.-F.; Xiao, J.-L.; Hu, Z.-L.; Chen, J.-G. SKF83959 Produces Antidepressant Effects in a Chronic Social Defeat Stress Model of Depression through BDNF-TrkB Pathway. Int. J. Neuropsychopharmacol. 2015, 18, pyu096. [Google Scholar] [CrossRef] [Green Version]
- Risinger, R.; Bhagwagar, Z.; Luo, F.; Cahir, M.; Miler, L.; Mendonza, A.E.; Meyer, J.H.; Zheng, M.; Hayes, W. Evaluation of safety and tolerability, pharmacokinetics, and pharmacodynamics of BMS-820836 in healthy subjects: A placebo-controlled, ascending single-dose study. Psychopharmacology 2013, 231, 2299–2310. [Google Scholar] [CrossRef]
- Bhagwagar, Z.; Torbeyns, A.; Hennicken, D.; Zheng, M.; Dunlop, B.W.; Mathew, S.J.; Khan, A.; Weisler, R.; Nelson, C.; Shelton, R.; et al. Assessment of the Efficacy and Safety of BMS-820836 in Patients with Treatment-Resistant Major Depression. J. Clin. Psychopharmacol. 2015, 35, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K. A Brief Review of the Pharmacology of Amitriptyline and Clinical Outcomes in Treating Fibromyalgia. Biomedicines 2017, 5, 24. [Google Scholar] [CrossRef]
- Deecher, D.C.; Beyer, C.E.; Johnston, G.; Bray, J.; Shah, S.; Abou-Gharbia, M.; Andree, T.H. Desvenlafaxine Succinate: A New Serotonin and Norepinephrine Reuptake Inhibitor. Experiment 2006, 318, 657–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraczewski, J.; Aedma, K.K. Tricyclic antidepressants. StatPearls (Internet). Available online: https://www.ncbi.nlm.nih.gov/books/NBK557791/ (accessed on 27 December 2022).
- Ferguson, J.M. SSRI Antidepressant Medications: Adverse effects and tolerability. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 22–27. [Google Scholar] [CrossRef]
- Haddad, P. Do antidepressants have any potential to cause addiction? J. Psychopharmacol. 1999, 13, 300–307. [Google Scholar] [CrossRef]
- Whiskey, E.; Taylor, D. A review of the adverse effects and safety of noradrenergic antidepressants. J. Psychopharmacol. 2013, 27, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Peitl, V.; Vlahović, D. Ansofaxine Hydrochloride. Arch. Psychiatry Res. 2020, 57, 87–90. [Google Scholar] [CrossRef]
- Mi, W.; Yang, F.; Li, H.; Xu, X.; Li, L.; Tan, Q.; Wang, G.; Zhang, K.; Tian, F.; Luo, J.; et al. Efficacy, Safety, and Tolerability of Ansofaxine (LY03005) Extended-Release Tablet for Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled, Dose-Finding, Phase 2 Clinical Trial. Int. J. Neuropsychopharmacol. 2021, 25, 252–260. [Google Scholar] [CrossRef]
- Luye Pharma. Luye Pharma’s Class 1 Innovative Antidepressant Ruoxinlin® Approved for Launch in China. Available online: https://www.luye.cn/lvye_en/view.php?id=2108 (accessed on 26 December 2022).
- Luye Pharma. Marketing Authorization Application Accepted by CDE for Luye Pharma’s Antidepressant Anshu-Faxine Hydrochloride Extended-Release Tablets. Available online: https://www.luye.cn/lvye_en/view.php?id=1954 (accessed on 26 December 2022).
- Luye Pharma. NDA Filing for Luye Pharma’s Antidepressant Drug LY03005 Accepted by the U.S. FDA. Available online: https://www.luye.cn/lvye_en/view.php?id=1809 (accessed on 26 December 2022).
- Fasipe, O.J. The emergence of new antidepressants for clinical use: Agomelatine paradox versus other novel agents. IBRO Rep. 2019, 6, 95–110. [Google Scholar] [CrossRef]
- Park, S.-C. Neurogenesis and antidepressant action. Cell Tissue Res. 2019, 377, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Roohi, E.; Jaafari, N.; Hashemian, F. On inflammatory hypothesis of depression: What is the role of IL-6 in the middle of the chaos? J. Neuroinflammation 2021, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Ishino, Y.; Shimizu, S.; Tohyama, M. Involvement of inflammatory responses in the brain to the onset of major depressive disorder due to stress exposure. Front. Aging Neurosci. 2022, 14, 934346. [Google Scholar] [CrossRef]
- Elias, E.; Zhang, A.Y.; Manners, M.T. Novel Pharmacological Approaches to the Treatment of Depression. Life 2022, 12, 196. [Google Scholar] [CrossRef]
- Richardson, B.; MacPherson, A.; Bambico, F. Neuroinflammation and neuroprogression in depression: Effects of alternative drug treatments. Brain Behav. Immun.-Health 2022, 26, 100554. [Google Scholar] [CrossRef]
- Brigitta, B. Pathophysiology of depression and mechanisms of treatment. Dialog. Clin. Neurosci. 2002, 4, 7–20. [Google Scholar] [CrossRef]
- Nemeroff, C.B. The State of Our Understanding of the Pathophysiology and Optimal Treatment of Depression: Glass Half Full or Half Empty? Am. J. Psychiatry 2020, 177, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Touya, M.; Lawrence, D.F.; Kangethe, A.; Chrones, L.; Evangelatos, T.; Polson, M. Incremental burden of relapse in patients with major depressive disorder: A real-world, retrospective cohort study using claims data. BMC Psychiatry 2022, 22, 152. [Google Scholar] [CrossRef]
- Kurimoto, N.; Inagaki, T.; Aoki, T.; Kadotani, H.; Kurimoto, F.; Kuriyama, K.; Yamada, N.; Ozeki, Y. Factors causing a relapse of major depressive disorders following successful electroconvulsive therapy: A retrospective cohort study. World J. Psychiatry 2021, 11, 841–853. [Google Scholar] [CrossRef]
- Schöpfel, J.; Farace, D.J. Grey literature. In Encyclopedia of Library and Information Sciences, 3rd ed.; Bates, M.J., Maack, M.N., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 2029–2039. [Google Scholar]
- Li, C.; Jiang, W.; Gao, Y.; Lin, F.; Zhu, H.; Wang, H.; Ye, L.; Qi, J.G.; Tian, J. Acute, subchronic oral toxicity, and genotoxicity evaluations of LPM570065, a new potent triple reuptake inhibitor. Regul. Toxicol. Pharmacol. 2018, 98, 129–139. [Google Scholar] [CrossRef]
- Guo, W.; Gao, Y.; Jiang, W.; Li, C.; Lin, F.; Zhu, H.; Wang, H.; Ye, L.; Qi, J.G.; Cen, X.; et al. Toxicity effects of a novel potent triple reuptake inhibitor, LPM570065, on the fertility and early embryonic development in Sprague-Dawley rats. Regul. Toxicol. Pharmacol. 2018, 100, 45–51. [Google Scholar] [CrossRef]
- Zhang, R.; Li, X.; Shi, Y.; Shao, Y.; Sun, K.; Wang, A.; Sun, F.; Liu, W.; Wang, D.; Jin, J.; et al. The Effects of LPM570065, a Novel Triple Reuptake Inhibitor, on Extracellular Serotonin, Dopamine and Norepinephrine Levels in Rats. PLoS ONE 2014, 9, e91775. [Google Scholar] [CrossRef] [Green Version]
- Meng, P.; Li, C.; Duan, S.; Ji, S.; Xu, Y.; Mao, Y.; Wang, H.; Tian, J. Epigenetic Mechanism of 5-HT/NE/DA Triple Reuptake Inhibitor on Adult Depression Susceptibility in Early Stress Mice. Front. Pharmacol. 2022, 13, 848251. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, W.; Sha, C.; Guo, W.; Li, C.; Zhao, F.; Wang, H.; Jiang, W.; Tian, J. Pharmacological Characterization of Toludesvenlafaxine as a Triple Reuptake Inhibitor. Front. Pharmacol. 2021, 12, 741794. [Google Scholar] [CrossRef] [PubMed]
- Luye Pharma. Luye Pharma’s Class 1 New Drug Anshufaxine Hydrochloride Extended-Release Tablets Meets Pre-Defined Endpoints in Phase III Trial. March 2021. Available online: https://www.luye.cn/lvye_en/view.php?id=1922 (accessed on 26 December 2022).
- National Library of Medicine (U.S.). Multiple Ascending Dose Study in Healthy Subjects to Evaluate the Safety, Tolerability, and Pharmacokinetics of LY03005. Available online: https://clinicaltrials.gov/ct2/show/NCT02271412 (accessed on 26 December 2022).
- National Library of Medicine (U.S.). Safety, Tolerability and Pharmacokinetics Study of LY03005 (LY03005SAD). Available online: https://clinicaltrials.gov/ct2/show/NCT02055300 (accessed on 26 December 2022).
- FiercePharma. Completion of Phase 1 Clinical Studies of Ansofaxine Hydrochloride Extended. 30 March 2015. Available online: https://www.fiercepharma.com/pharma-asia/completion-of-phase-1-clinical-studies-of-ansofaxine-hydrochloride-extended-release (accessed on 27 December 2022).
- Chua, S. Phase I Study Program for Ansofaxine (LY03005) Is Completed in USA. Available online: https://www.linkedin.com/pulse/phase-i-study-program-ansofaxine-ly03005-completed-usa-sam-chua (accessed on 27 December 2022).
- National Library of Medicine (U.S.). Pilot BA Study of New LY03005 vs. Pristiq. Available online: https://clinicaltrials.gov/ct2/show/NCT02988024 (accessed on 26 December 2022).
- National Library of Medicine (U.S.). A Relative Bioavailability Food Effect Study of LY03005. Available online: https://clinicaltrials.gov/ct2/show/NCT03822065 (accessed on 26 December 2022).
- National Library of Medicine (U.S.). A Study of LY03005 vs. Pristiq. Available online: https://clinicaltrials.gov/ct2/show/NCT03733574 (accessed on 26 December 2022).
- National Library of Medicine (U.S.). Relative Bioavailability (RBA) Study of LY03005 vs. Pristiq. Available online: https://clinicaltrials.gov/ct2/show/NCT03357796 (accessed on 26 December 2022).
- National Library of Medicine (U.S.). Dose-Finding Clinical Trial to Evaluate the Efficacy and Safety of LY03005 Extended-Release Tablets in the Treatment of Major Depressive Disorder (MDD). Available online: https://clinicaltrials.gov/ct2/show/NCT03785652 (accessed on 26 December 2022).
- National Library of Medicine (U.S.). A Study to Evaluate the Efficacy and Safety of Annsofaxine Hydrochloride Extended-Release Tablets in the Treatment of Major Depressive Disorder (MDD). Available online: https://clinicaltrials.gov/ct2/show/NCT04853407 (accessed on 26 December 2022).
- Greenberg, P.E.; Fournier, A.-A.; Sisitsky, T.; Simes, M.; Berman, R.; Koenigsberg, S.H.; Kessler, R.C. The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). Pharmacoeconomics 2021, 39, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.-A.; Hensel, J.; Davies, P.E.; Brown, L.; DeChairo, B.M.; Mulsant, B.H. Economic Burden of Depression and Associated Resource Use in Manitoba, Canada. Can. J. Psychiatry 2019, 65, 338–346. [Google Scholar] [CrossRef]
- Touloumis, C. The burden and the challenge of treatment-resistant depression. Psychiatriki 2021, 32 (Suppl. SI), 11–14. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, D.H.; Rive, B.; Denee, T.R. The humanistic and economic burden of treatment-resistant depression in Europe: A cross-sectional study. BMC Psychiatry 2019, 247, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masand, P.S. Tolerability and adherence issues in antidepressant therapy. Clin. Ther. 2003, 25, 2289–2304. [Google Scholar] [CrossRef]
- Ho, S.C.; Jacob, S.A.; Tangiisuran, B. Barriers and facilitators of adherence to antidepressants among outpatients with major depressive disorder: A qualitative study. PLoS ONE 2017, 12, e0179290. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.; LaPalombara, Z.; Ahmari, S.E. Monoamine abnormalities in the SAPAP3 knockout model of obsessive-compulsive disorder-related behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calandre, E.P.; Rico-Villademoros, F.; Slim, M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin. Pharmacother. 2015, 16, 1347–1368. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.S.; Bell, C.E.; Pollard, D.A.; Bell, J.C.E. Revisiting the Monoamine Hypothesis of Depression: A New Perspective. Perspect. Med. Chem. 2014, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Liu, J.; Wang, M.; Zhang, Y.; Li, L. From Serotonin to Neuroplasticity: Evolvement of Theories for Major Depressive Disorder. Front. Cell. Neurosci. 2017, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Massart, R.; Mongeau, R.; Lanfumey, L. Beyond the monoaminergic hypothesis: Neuroplasticity and epigenetic changes in a transgenic mouse model of depression. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2485–2494. [Google Scholar] [CrossRef] [Green Version]
- Epperson, C.N.; Rubinow, D.R.; Meltzer-Brody, S.; Deligiannidis, K.M.; Riesenberg, R.; Krystal, A.D.; Bankole, K.; Huang, M.-Y.; Li, H.; Brown, C.; et al. Effect of brexanolone on depressive symptoms, anxiety, and insomnia in women with postpartum depression: Pooled analyses from 3 double-blind, randomized, placebo-controlled clinical trials in the HUMMINGBIRD clinical program. J. Affect. Disord. 2023, 320, 353–359. [Google Scholar] [CrossRef]
- Cooper, M.C.; Kilvert, H.S.; Hodgkins, P.; Roskell, N.S.; Eldar-Lissai, A. Using Matching-Adjusted Indirect Comparisons and Network Meta-analyses to Compare Efficacy of Brexanolone Injection with Selective Serotonin Reuptake Inhibitors for Treating Postpartum Depression. CNS Drugs 2019, 33, 1039–1052. [Google Scholar] [CrossRef] [Green Version]
- Bahji, A.; Vazquez, G.H.; Zarate, C.A. Comparative efficacy of racemic ketamine and esketamine for depression: A systematic review and meta-analysis. J. Affect. Disord. 2020, 278, 542–555. [Google Scholar] [CrossRef]
- Xiong, J.; Lipsitz, O.; Chen-Li, D.; Rosenblat, J.D.; Rodrigues, N.B.; Carvalho, I.; Lui, L.M.; Gill, H.; Narsi, F.; Mansur, R.B.; et al. The acute antisuicidal effects of single-dose intravenous ketamine and intranasal esketamine in individuals with major depression and bipolar disorders: A systematic review and meta-analysis. J. Psychiatr. Res. 2020, 134, 57–68. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiliu, O. Efficacy, Tolerability, and Safety of Toludesvenlafaxine for the Treatment of Major Depressive Disorder—A Narrative Review. Pharmaceuticals 2023, 16, 411. https://doi.org/10.3390/ph16030411
Vasiliu O. Efficacy, Tolerability, and Safety of Toludesvenlafaxine for the Treatment of Major Depressive Disorder—A Narrative Review. Pharmaceuticals. 2023; 16(3):411. https://doi.org/10.3390/ph16030411
Chicago/Turabian StyleVasiliu, Octavian. 2023. "Efficacy, Tolerability, and Safety of Toludesvenlafaxine for the Treatment of Major Depressive Disorder—A Narrative Review" Pharmaceuticals 16, no. 3: 411. https://doi.org/10.3390/ph16030411
APA StyleVasiliu, O. (2023). Efficacy, Tolerability, and Safety of Toludesvenlafaxine for the Treatment of Major Depressive Disorder—A Narrative Review. Pharmaceuticals, 16(3), 411. https://doi.org/10.3390/ph16030411