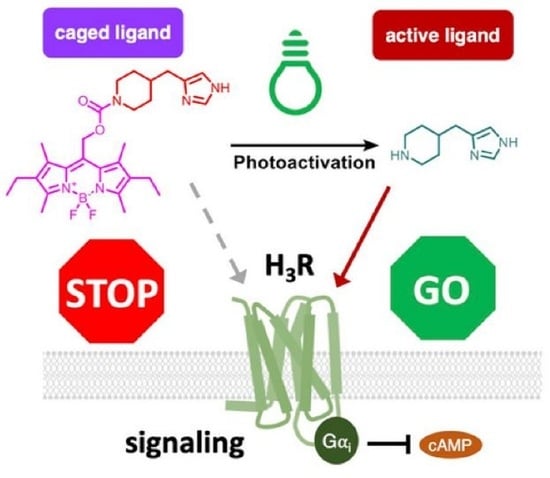
Synthesis and Pharmacological Characterization of New Photocaged Agonists for Histamine H3 and H4 Receptors
Abstract
:1. Introduction
2. Results
2.1. Design, Synthesis, and Photochemical Characterization of Photocaged Compounds
2.2. Photopharmacological Characterization
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. (2,8-Diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-4λ4,5λ4-dipyrrolo [1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)methyl acetate, 2
4.1.2. (2,8-Diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-4λ4,5λ4-dipyrrolo [1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)methanol, 3
4.1.3. (2,8-Diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-4λ4,5λ4-dipyrrolo [1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)methyl (4-nitrophenyl) carbonate, 4
4.1.4. (2,8-Diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-4λ4,5λ4-dipyrrolo [1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)methyl 4-((1H-imidazol-4-yl)methyl)piperidine-1-carboxylate, 5
4.1.5. (2,8-Diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-4λ4,5λ4-dipyrrolo [1,2-c:2′,1′-f][1,3,2]diazaborinin-10-yl)methyl (2-(5-methyl-1H-imidazol-4-yl)ethyl)carbamate, 6
4.2. Nephelometry
4.3. (Photo)chemical Stability Assay
4.4. Materials for Pharmacological Evaluation
4.5. Cell Culture and Transfection
4.6. Membrane Preparation
4.7. Radioligand Binding Assay
4.8. cAMP Inhibition by FRET-EPAC Biosensor
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuchter, M.J. On the Promise of Photopharmacology Using Photoswitches: A Medicinal Chemist’s Perspective. J. Med. Chem. 2020, 63, 11436–11447. [Google Scholar] [CrossRef]
- Silva, J.M.; Silva, E.; Reis, R.L. Light-triggered release of photocaged therapeutics—Where are we now? J. Control. Release 2019, 298, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Welleman, I.M.; Hoorens, M.W.H.; Feringa, B.L.; Boersma, H.H.; Szymanski, W. Photoresponsive molecular tools for emerging applications of light in medicine. Chem. Sci. 2020, 11, 11672–11691. [Google Scholar] [CrossRef] [PubMed]
- Wijtmans, M.; Josimovic, I.; Vischer, H.F.; Leurs, R. Optical control of Class A G protein-coupled receptors with photoswitchable ligands. Curr. Opin. Pharmacol. 2022, 63, 102192. [Google Scholar] [CrossRef] [PubMed]
- Stacko, P.; Solomek, T. Photoremovable Protecting Groups: Across the Light Spectrum to Near- Infrared Absorbing Photocages. Chimia 2021, 75, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Bojtár, M.; Németh, K.; Domahidy, F.; Knorr, G.; Verkman, A.; Kállay, M.; Kele, P. Conditionally Activatable Visible-Light Photocages. J. Am. Chem. Soc. 2020, 142, 15164–15171. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, M.; Wang, F.; Lu, S.; Chen, X. Recent progress in studies of photocages. Smart Mol. 2023, 1, e20220003. [Google Scholar] [CrossRef]
- Monteiro, D.C.F.; Amoah, E.; Rogers, C.; Pearson, A.R. Using photocaging for fast time-resolved structural biology studies. Acta Crystallogr. D Struct. Biol. 2021, 77, 1218–1232. [Google Scholar] [CrossRef] [PubMed]
- Leurs, R.; Vischer, H.F.; Wijtmans, M.; de Esch, I.J. En route to new blockbuster anti-histamines: Surveying the offspring of the expanding histamine receptor family. Trends Pharmacol. Sci. 2011, 32, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Chazot, P.L.; Cowart, M.; Gutzmer, R.; Leurs, R.; Liu, W.L.; Stark, H.; Thurmond, R.L.; Haas, H.L. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol. Rev. 2015, 67, 601–655. [Google Scholar] [CrossRef] [PubMed]
- Passani, M.B.; Blandina, P. Histamine receptors in the CNS as targets for therapeutic intervention. Trends Pharmacol. Sci. 2011, 32, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Ghamari, N.; Zarei, O.; Arias-Montano, J.A.; Reiner, D.; Dastmalchi, S.; Stark, H.; Hamzeh-Mivehroud, M. Histamine H3 receptor antagonists/inverse agonists: Where do they go? Pharmacol. Ther. 2019, 200, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Alamilla, G.; Marquez-Gomez, R.; Garcia-Galvez, A.M.; Morales-Figueroa, G.E.; Arias-Montano, J.A. The Histamine H3 Receptor: Structure, Pharmacology, and Function. Mol. Pharmacol. 2016, 90, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Krief, S.; Berrebi-Bertrand, I.; Nagmar, I.; Giret, M.; Belliard, S.; Perrin, D.; Uguen, M.; Robert, P.; Lecomte, J.M.; Schwartz, J.C.; et al. Pitolisant, a wake-promoting agent devoid of psychostimulant properties: Preclinical comparison with amphetamine, modafinil, and solriamfetol. Pharmacol. Res. Perspect. 2021, 9, e00855. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, N.; Okuampa, D.; Hansen, H.; Alvarez, M.; Cornett, E.M.; Kakazu, J.; Kaye, A.M.; Kaye, A.D. pitolisant, a novel histamine-3 receptor competitive antagonist, and inverse agonist, in the treatment of excessive daytime sleepiness in adult patients with narcolepsy. Health Psychol. Res. 2022, 10, 34222. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Yang, S.; Wang, T.; Xu, Z.; Xu, J.; Gao, H.; Chen, G. Pitolisant versus placebo for excessive daytime sleepiness in narcolepsy and obstructive sleep apnea: A meta-analysis from randomized controlled trials. Pharmacol. Res. 2021, 167, 105522. [Google Scholar] [CrossRef]
- Schirmer, B.; Neumann, D. The Function of the Histamine H4 Receptor in Inflammatory and Inflammation-Associated Diseases of the Gut. Int. J. Mol. Sci. 2021, 22, 6116. [Google Scholar] [CrossRef] [PubMed]
- Schaper-Gerhardt, K.; Rossbach, K.; Nikolouli, E.; Werfel, T.; Gutzmer, R.; Mommert, S. The role of the histamine H4 receptor in atopic dermatitis and psoriasis. Br. J. Pharmacol. 2020, 177, 490–502. [Google Scholar] [CrossRef]
- Shan, L.; Martens, G.J.M.; Swaab, D.F. Histamine-4 Receptor: Emerging Target for the Treatment of Neurological Diseases. Curr. Top. Behav. Neurosci. 2022, 59, 131–145. [Google Scholar] [CrossRef]
- Lavis, L.D.; Raines, R.T. Bright building blocks for chemical biology. ACS Chem. Biol. 2014, 9, 855–866. [Google Scholar] [CrossRef]
- Shrestha, P.; Kand, D.; Weinstain, R.; Winter, A.H. meso-Methyl BODIPY Photocages: Mechanisms, Photochemical Properties, and Applications. J. Am. Chem. Soc. 2023, 145, 17497–17514. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.D.; van Rijn, R.M.; Ling, P.; Bakker, R.A.; Thurmond, R.L.; Leurs, R. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: Identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. J. Pharmacol. Exp. Ther. 2005, 314, 1310–1321. [Google Scholar] [CrossRef]
- Vollinga, R.C.; de Koning, J.P.; Jansen, F.P.; Leurs, R.; Menge, W.M.; Timmerman, H. A new potent and selective histamine H3 receptor agonist, 4-(1H-imidazol-4-ylmethyl)piperidine. J. Med. Chem. 1994, 37, 332–333. [Google Scholar] [CrossRef]
- Kitbunnadaj, R.; Hashimoto, T.; Poli, E.; Zuiderveld, O.P.; Menozzi, A.; Hidaka, R.; de Esch, I.J.; Bakker, R.A.; Menge, W.M.; Yamatodani, A.; et al. N-substituted piperidinyl alkyl imidazoles: Discovery of methimepip as a potent and selective histamine H3 receptor agonist. J. Med. Chem. 2005, 48, 2100–2107. [Google Scholar] [CrossRef]
- Ishikawa, M.; Furuuchi, T.; Yamauchi, M.; Yokoyama, F.; Kakui, N.; Sato, Y. Synthesis and structure-activity relationships of N-aryl-piperidine derivatives as potent (partial) agonists for human histamine H3 receptor. Bioorg. Med. Chem. 2010, 18, 5441–5448. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, W.D.; Sher, R.; Berlin, M.; Shih, N.Y.; Aslanian, R.; Schwerdt, J.H.; McCormick, K.D.; Piwinski, J.J.; West, R.E., Jr.; Anthes, J.C.; et al. Novel histamine H3 receptor antagonists based on the 4-[(1H-imidazol-4-yl)methyl]piperidine scaffold. Bioorg. Med. Chem. Lett. 2006, 16, 395–399. [Google Scholar] [CrossRef]
- Im, D.; Kishikawa, J.I.; Shiimura, Y.; Hisano, H.; Ito, A.; Fujita-Fujiharu, Y.; Sugita, Y.; Noda, T.; Kato, T.; Asada, H.; et al. Structural insights into the agonists binding and receptor selectivity of human histamine H4 receptor. Nat. Commun. 2023, 14, 6538. [Google Scholar] [CrossRef]
- Xia, R.; Shi, S.; Xu, Z.; Vischer, H.F.; Windhorst, A.D.; Qian, Y.; Duan, Y.; Liang, J.; Chen, K.; Zhang, A.; et al. Structural basis of ligand recognition and design of antihistamines targeting histamine H4 receptor. Nat. Commun. 2024, 15, 2493. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, A.; Deiters, A. Development of photolabile protecting groups and their application to the optochemical control of cell signaling. Curr. Opin. Struct. Biol. 2019, 57, 164–175. [Google Scholar] [CrossRef]
- Ellis-Davies, G.C.R. Useful Caged Compounds for Cell Physiology. Acc. Chem. Res. 2020, 53, 1593–1604. [Google Scholar] [CrossRef]
- Jia, S.; Sletten, E.M. Spatiotemporal Control of Biology: Synthetic Photochemistry Toolbox with Far-Red and Near-Infrared Light. ACS Chem. Biol. 2022, 17, 3255–3269. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.P.; Syed, A.; Beck, C.L.; Albright, T.R.; Mahoney, K.M.; Unash, R.; Smith, E.A.; Winter, A.H. BODIPY-derived photoremovable protecting groups unmasked with green light. J. Am. Chem. Soc. 2015, 137, 3783–3786. [Google Scholar] [CrossRef] [PubMed]
- Amat-Guerri, F.; Liras, M.; Carrascoso, M.L.; Sastre, R. Methacrylate-tethered analogs of the laser dye PM567--synthesis, copolymerization with methyl methacrylate and photostability of the copolymers. Photochem. Photobiol. 2003, 77, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Egodawaththa, N.M.; Guruge, C.; Marquez, O.A.V.; Likes, M.; Nesnas, N. Blue and Green Light Responsive Caged Glutamate. J. Photochem. Photobiol. A Chem. 2024, 447, 115183. [Google Scholar] [CrossRef]
- Rubinstein, N.; Liu, P.; Miller, E.W.; Weinstain, R. meso-Methylhydroxy BODIPY: A scaffold for photo-labile protecting groups. Chem. Commun. 2015, 51, 6369–6372. [Google Scholar] [CrossRef] [PubMed]
- Mocking, T.A.M.; Verweij, E.W.E.; Vischer, H.F.; Leurs, R. Homogeneous, Real-Time NanoBRET Binding Assays for the Histamine H3 and H4 Receptors on Living Cells. Mol. Pharmacol. 2018, 94, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Dekker, M.E.; Leurs, R.; Vischer, H.F. Pharmacological characterization of seven human histamine H3 receptor isoforms. Eur. J. Pharmacol. 2024, 968, 176450. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.H.; Forbush, B., 3rd; Hoffman, J.F. Rapid photolytic release of adenosine 5’-triphosphate from a protected analogue: Utilization by the Na:K pump of human red blood cell ghosts. Biochemistry 1978, 17, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Xu, Y.; Kim, B.; Rha, H.; Zhang, B.; Li, M.; Yang, G.-F.; Kim, J.S. Photo-controllable biochemistry: Exploiting the photocages in phototherapeutic window. Chem 2023, 9, 29–64. [Google Scholar] [CrossRef]
- Klan, P.; Solomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable protecting groups in chemistry and biology: Reaction mechanisms and efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef]
- Kand, D.; Liu, P.; Navarro, M.X.; Fischer, L.J.; Rousso-Noori, L.; Friedmann-Morvinski, D.; Winter, A.H.; Miller, E.W.; Weinstain, R. Water-Soluble BODIPY Photocages with Tunable Cellular Localization. J. Am. Chem. Soc. 2020, 142, 4970–4974. [Google Scholar] [CrossRef] [PubMed]
- Roessler, W.G.; Brewer, C.R. Permanent turbidity standards. Appl. Microbiol. 1967, 15, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, J.M.; Vedel, L.; Brauner-Osborne, H. cAMP biosensors applied in molecular pharmacological studies of G protein-coupled receptors. Methods Enzymol. 2013, 522, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Scharf, M.M.; Zimmermann, M.; Wilhelm, F.; Stroe, R.; Waldhoer, M.; Kolb, P. A Focus on Unusual ECL2 Interactions Yields β2-Adrenergic Receptor Antagonists with Unprecedented Scaffolds. ChemMedChem 2020, 15, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.A.; Cutrone, E.C.; Kotenko, S.V.; Krause, C.D.; Langer, J.A. Modifications of vectors pEF-BOS, pcDNA1 and pcDNA3 result in improved convenience and expression. Biotechniques 1996, 21, 1013–1015. [Google Scholar] [CrossRef]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef]




| Compound | pKi | pEC50 | ||
|---|---|---|---|---|
| In Dark | +560 nm | In Dark | +560 nm | |
| VUF25657 (5) | 7.3 ± 0.6 (4) | 8.4 ± 0.2 (4) * | 8.0 ± 0.4 (4) | 9.3 ± 0.2 (4) * |
| immepip | 9.4 ± 0.2 (4) | 9.4 ± 0.3 (4) | 10.5 ± 0.6 (4) | 10.2 ± 0.6 (4) |
| VUF25678 (6) | <6 (3) | <6 (3) | N.D. | N.D. |
| 4-methylhistamine | 7.2 ± 0.2 (3) | 7.2 ± 0.1 (3) | N.D. | N.D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Gao, M.; Wijtmans, M.; Vischer, H.F.; Leurs, R. Synthesis and Pharmacological Characterization of New Photocaged Agonists for Histamine H3 and H4 Receptors. Pharmaceuticals 2024, 17, 536. https://doi.org/10.3390/ph17040536
Zheng Y, Gao M, Wijtmans M, Vischer HF, Leurs R. Synthesis and Pharmacological Characterization of New Photocaged Agonists for Histamine H3 and H4 Receptors. Pharmaceuticals. 2024; 17(4):536. https://doi.org/10.3390/ph17040536
Chicago/Turabian StyleZheng, Yang, Meichun Gao, Maikel Wijtmans, Henry F. Vischer, and Rob Leurs. 2024. "Synthesis and Pharmacological Characterization of New Photocaged Agonists for Histamine H3 and H4 Receptors" Pharmaceuticals 17, no. 4: 536. https://doi.org/10.3390/ph17040536
APA StyleZheng, Y., Gao, M., Wijtmans, M., Vischer, H. F., & Leurs, R. (2024). Synthesis and Pharmacological Characterization of New Photocaged Agonists for Histamine H3 and H4 Receptors. Pharmaceuticals, 17(4), 536. https://doi.org/10.3390/ph17040536