Marine Non-Glycosaminoglycan Sulfated Glycans as Potential Pharmaceuticals
Abstract
:1. Introduction
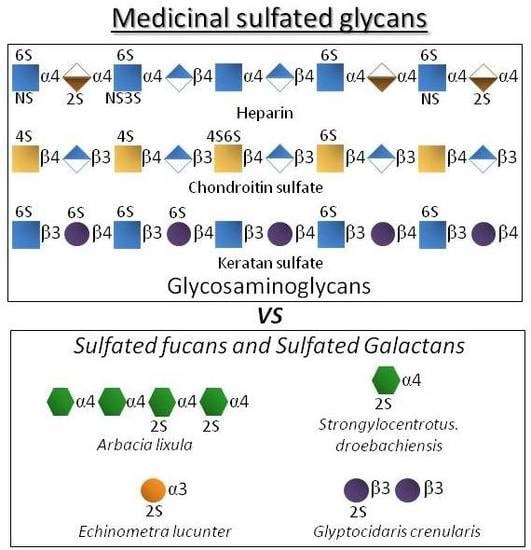
2. Structure


| Polysaccharide Type | Molecular Type | Structural Units | Overall Aspects | Medical Benefits |
|---|---|---|---|---|
| GAGs | Heparin | IdoA2S + GlcNS6S | As the most negatively charged biomacromolecule in nature, it interacts and regulates various protein types. | Potent anticoagulant and antithrombotic agent [3,4]. |
| Chondroitin sulfate | GlcA + GalNAc(4S and/or 6S) | The most abundant GAG of the body and of connective (cartilage) tissues. | Used in cases of osteoarthritis, osteoarthrosis and sometimes osteoporosis [5,6,7]. | |
| Keratan sulfate | Gal + GlcNAc (6S at both units but more often at GlcNAc) | Highly abundant in cornea. Related with the proper visual functions. | Explored as functional ingredient in eye-drops [8,9]. | |
| Marine glycans | SFs | Fuc (2S and/or 4S) | Found in well-defined structures in sea urchins and sea cucumbers. | Exhibits potential effects in anticoagulation, antithrombosis, anti-angiogenesis, antitumor, anti-inflammation and antimicrobial infections [14,15,16]. |
| SGs | Gal (2S and/or 3S, and/or 4S) | Found in well-defined structures in red algae, sea urchins and ascidians. |
3. Medical Mechanisms of Action
3.1. Anticoagulant/Antithrombotic Mechanisms of Action
3.1.1. The Serpin-Dependent Mechanisms
| Polysaccharide Type | Source | aPTT (units/mg) a | IC50 (μg/mL) | ||
|---|---|---|---|---|---|
| IIa/AT | IIa/HCII | Xa/AT | |||
| Invertebrate 3-linked α-l-SF | S. purpuratus I | 76 | 0.3 | 0.3 | 2 |
| S. purpuratus II | 10 | 0.9 | 2 | nd b | |
| S. pallidus | 18 | >500 | >500 | >500 | |
| L. variegatus I | 3 | >500 | >500 | >500 | |
| S. franciscanus | ~2 | >500 | >500 | 250 | |
| L. grisea | <1 | >500 | >500 | >500 | |
| Invertebrate 4-linked α-l-SF | S. droebachiensis | <1 | nd | nd | nd |
| A. lixula | ~2 | 150 | 150 | >500 | |
| Invertebrate α-l-SG | E. lucunter | 20 | 3 | 6 | 20 |
| G. crenularis | <1 | nd | nd | Nd | |
| Red algal SGs | B. occidentalis | 93 | 0.02 | 1.1 | 2.5 |
| G. crinale | 65 | 0.02 | 25 | 1.5 | |
3.1.2. The Serpin-Independent Mechanism

3.2. Anti-Angiogenic and Anticancer Mechanisms of Action
3.3. Anti-Inflammatory Mechanisms of Action

3.4. Antimicrobial Mechanism of Action

4. Concluding Remarks and Existing Challenges in Drug Development
| Medical System | GAGs | Marine Sulfated Glycans |
|---|---|---|
| Anticoagulation and antitrombosis | Serpin-dependent mechanism
| Serpin-dependent mechanism
|
| Anti-angiogenesis and anticancer |
| |
| Anti-inflammation |
| |
| Antimicrobial infections |
| |
Conflicts of Interest
References
- Volpi, N. Therapeutic applications of glycosaminoglycans. Curr. Med. Chem. 2006, 13, 1799–1810. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. A dilemma in the glycosaminoglycan-based therapy: Synthetic or naturally unique molecules? Med. Res. Rev. 2015, 35, 1195–1219. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.; Hogwood, J.; Mulloy, B. The anticoagulant and antithrombotic mechanisms of heparin. Handb. Exp. Pharmacol. 2012, 207, 43–61. [Google Scholar] [PubMed]
- Hirsh, J.; Anand, S.S.; Halperin, J.L.; Fuster, V. Guide to anticoagulant therapy: Heparin: A statement for healthcare professionals from the American Heart Association. Circulation 2001, 103, 2994–3018. [Google Scholar] [CrossRef] [PubMed]
- Uebelhart, D. Clinical review of chondroitin sulfate in osteoarthritis. Osteoarthritis Cartilage 2008, 16, S19–S21. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T. Anti-arthrosis treatments: Efficacy and tolerance of chondroitin sulfates (CS 4&6). Presse Med. 1998, 27, 1862–1865. [Google Scholar] [PubMed]
- Pomin, V.H.; Piquet, A.A.; Pereira, M.S.; Mourão, P.A. Residual keratan sulfate in chondroitin sulfate formulations for oral administration. Carbohydr. Polym. 2012, 90, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Keratan sulfate: An up-to-date review. Int. J. Biol. Macromol. 2015, 72, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Morikawa, K.; Tawada, A.; Miyauchi, S.; Yoshida, K.; Asari, A. Keratan Sulfate Oligosaccharide Fraction and Pharmaceutical Containing the Same. U.S. Patent 6,159,954 A, 12 December 2000. [Google Scholar]
- Crowther, M.A.; Warkentin, T.E. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood 2008, 111, 4871–4879. [Google Scholar] [CrossRef] [PubMed]
- Babikian, V.L.; Kase, C.S.; Pessin, M.S.; Norrving, B.; Gorelick, P.B. Intracerebral hemorrhage in stroke patients anticoagulated with heparin. Stroke 1989, 20, 1500–1503. [Google Scholar] [CrossRef] [PubMed]
- Kelton, J.G.; Hirsh, J. Bleeding associated with antithrombotic therapy. Semin. Hematol. 1980, 17, 259–379. [Google Scholar] [PubMed]
- Funderburgh, J.L. Keratan sulfate: Structure, biosynthesis, and function. Glycobiology 2000, 10, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Mourão, P.A. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Fucanomics and galactanomics: marine distribution, medicinal impact, conceptions, and challenges. Mar. Drugs 2012, 10, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Fucanomics and galactanomics: Current status in drug discovery, mechanisms of action and role of the well-defined structures. Biochim. Biophys. Acta 2012, 1820, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Glauser, B.F.; Rezende, R.M.; Melo, F.R.; Pereira, M.S.; Francischetti, I.M.; Monteiro, R.Q.; Rezaie, A.R.; Mourão, P.A. Anticoagulant activity of a sulfated galactan: Serpin-independent effect and specific interaction with factor Xa. Thromb. Haemost. 2009, 102, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Glauser, B.F.; Mourão, P.A.; Pomin, V.H. Marine sulfated glycans with serpin-unrelated anticoagulant properties. Adv. Clin. Chem. 2013, 62, 269–303. [Google Scholar] [PubMed]
- Quinderé, A.L.; Santos, G.R.; Oliveira, S.N.; Glauser, B.F.; Fontes, B.P.; Queiroz, I.N.; Benevides, N.M.; Pomin, V.H.; Mourão, P.A. Is the antithrombotic effect of sulfated galactans independent of serpin? J. Thromb. Haemost. 2014, 12, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Pereira, M.S.; Valente, A.P.; Tollefsen, D.M.; Pavão, M.S.; Mourão, P.A. Selective cleavage and anticoagulant activity of a sulfated fucan: stereospecific removal of a 2-sulfate ester from the polysaccharide by mild acid hydrolysis, preparation of oligosaccharides, and heparin cofactor II-dependent anticoagulant activity. Glycobiology 2005, 15, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A. Use of sulfated fucans as anticoagulant and antithrombotic agents: Future perspectives. Curr. Pharm. Des. 2004, 10, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Tollefsen, D.M.; Pestka, C.A.; Monafo, W.J. Activation of heparin cofactor II by dermatan sulfate. J. Biol. Chem. 1983, 258, 6713–6716. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.R.; Tovar, A.M.; Capillé, N.V.; Pereira, M.S.; Pomin, V.H.; Mourão, P.A. Structural and functional analyses of bovine and porcine intestinal heparins confirm they are different drugs. Drug Discov. Today 2014, 19, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I. Low-molecular-weight heparins. N. Engl. J. Med. 1992, 337, 688–698. [Google Scholar]
- Patnaik, M.M.; Moll, S. Inherited antithrombin deficiency: A review. Haemophilia 2008, 14, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.J.; Oliveira, S.N.; Melo, F.R.; Pereira, M.G.; Benevides, N.M.; Mourão, P.A. Slight differences in sulfation of algal galactans account for differences in their anticoagulant and venous antithrombotic activities. Thromb. Haemost. 2008, 99, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Sasisekharan, R.; Venkataraman, G. Heparin and heparan sulfate: Biosynthesis, structure and function. Curr. Opin. Chem. Biol. 2000, 4, 626–631. [Google Scholar] [CrossRef]
- Rabestein, D.L. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef]
- Pomin, V.H.; Mulloy, B. Current structural biology of the heparin interactome. Curr. Opin. Struct. Biol. 2015, 34, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Park, Y.; Huang, R.; Heiss, C.; Sharp, J.S.; Azadi, P.; Prestegard, J.H. Exploiting enzyme specificities in digestions of chondroitin sulfates A and C: Production of well-defined hexasaccharides. Glycobiology 2012, 22, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, S.; Yamada, S.; Sugahara, K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 2015, 34, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Pol. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed]
- Farias, E.H.; Pomin, V.H.; Valente, A.P.; Nader, H.B.; Rocha, H.A.; Mourão, P.A. A preponderantly 4-sulfated, 3-linked galactan from the green alga Codium isthmocladum. Glycobiology 2008, 18, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Structural and functional insights into sulfated galactans: A systematic review. Glycoconj. J. 2010, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, A.; Björk, I. Properties of antithrombin-thrombin complex formed in the presence and in the absence of heparin. Biochem. J. 1983, 213, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dewar, L.; Song, Y.; Kulczycky, M.; Blajchman, M.A.; Fenton, J.W., 2nd; Andrew, M.; Delorme, M.; Ginsberg, J.; Preissner, K.T.; et al. Inhibition of thrombin by antithrombin III and heparin cofactor II in vivo. Thromb. Haemost. 1995, 73, 405–412. [Google Scholar] [PubMed]
- Maaroufi, R.M.; Jozefowicz, M.; Tapon-Bretaudière, J.; Jozefonvicz, J.; Fischer, A.M. Mechanism of thrombin inhibition by heparin cofactor II in the presence of dermatan sulphates, native or oversulphated, and a heparin-like dextran derivative. Biomaterials 1997, 18, 359–366. [Google Scholar] [CrossRef]
- Tollefsen, D.M. Vascular dermatan sulfate and heparin cofactor II. Prog. Mol. Biol. Transl. Sci. 2010, 93, 351–372. [Google Scholar] [PubMed]
- Pomin, V.H. Anticoagulant motifs of marine sulfated glycans. Glycoconj. J. 2014, 31, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Mourão, P.A. Specific sulfation and glycosylation—a structural combination for the anticoagulation of marine carbohydrates. Front. Cell. Infect. Microbiol. 2014, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, M.; Xiao, C.; Yang, L.; Zhou, L.; Gao, N.; Li, Z.; Chen, J.; Chen, J.; Liu, J.; Qian, H.; Zhao, J. Discovery of an intrinsic tenase complex inhibitor: Pure nonasaccharide from fucosylated glycosaminoglycan. Proc. Natl. Acad. Sci. USA 2015, 112, 8284–8289. [Google Scholar] [CrossRef] [PubMed]
- Otrock, Z.K.; Mahfouz, R.A.; Makarem, J.A.; Shamseddine, A.I. Understanding the biology of angiogenesis: Review of the most important molecular mechanisms. Blood Cells Mol. Dis. 2007, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Faham, S.; Hileman, R.E.; Fromm, J.R.; Linhardt, R.J.; Rees, D.C. Heparin structure and interactions with basic fibroblast growth factor. Science 1996, 271, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Burke, D.F.; von Delft, F.; Mulloy, B.; Blundell, T.L. Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature 2000, 407, 1029–1034. [Google Scholar] [PubMed]
- Schlessinger, J.; Plotnikov, A.N.; Ibrahimi, O.A.; Eliseenkova, A.V.; Yeh, B.K.; Yayon, A.; Linhardt, R.J.; Mohammadi, M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell. 2000, 6, 743–750. [Google Scholar] [CrossRef]
- Goodger, S.J.; Robinson, C.J.; Murphy, K.J.; Gasiunas, N.; Harmer, N.J.; Blundell, T.L.; Pye, D.A.; Gallagher, J.T. Evidence that heparin saccharides promote FGF2 mitogenesis through two distinct mechanisms. J. Biol. Chem. 2008, 283, 13001–13008. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.J.; Harmer, N.J.; Goodger, S.J.; Blundell, T.L.; Gallagher, J.T. Cooperative dimerization of fibroblast growth factor 1 (FGF1) upon a single heparin saccharide may drive the formation of 2:2:1 FGF1.FGFR2c.heparin ternary complexes. J. Biol. Chem. 2005, 280, 42274–42282. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, M.; Hricovíni, M.; Torri, G. Ineraction of heparins with fibroblast growth factors: conformational aspects. Curr. Pharm. Des. 2007, 13, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Guglier, S.; Hricovíni, M.; Raman, R.; Polito, L.; Torri, G.; Casu, B.; Sasisekharan, R.; Guerrini, M. Minimum FGF2 binding structural requirements of heparin and heparan sulfate oligosaccharides as determined by NMR spectroscopy. Biochemistry 2008, 47, 13862–13869. [Google Scholar] [CrossRef] [PubMed]
- Teran, M.; Nugent, M.A. Synergistic binding of vascular endothelial growth factor-A and its receptors to heparin selectively modulates complex affinity. J. Biol. Chem. 2015, 290, 16451–16462. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Dai, C.; Peng, S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol. Cancer Res. 2011, 9, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Khosravi Shahi, P.; Soria Lovelle, A.; Pérez Manga, G. Tumoral angiogenesis and breast cancer. Clin. Transl. Oncol. 2009, 11, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Ushakova, N.A.; Usov, A.I.; Kiselevskiy, M.V.; Nifantiev, N.E. Fucoidans: Pro- or antiangiogenic agents? Glycobiology 2014, 24, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y. Fucoidan as a Marine anticancer agent in preclinical development. Mar. Drugs 2014, 12, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Zemani, F.; Benisvy, D.; Galy-Fauroux, I.; Lokajczyk, A.; Colliec-Jouault, S.; Uzan, G.; Fischer, A.M.; Boisson-Vidal, C. Low-molecular-weight fucoidan enhances the proangiogenic phenotype of endothelial progenitor cells. Biochem. Pharmacol. 2005, 15, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Dube, D.H.; Bertozzi, C.R. Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005, 4, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.B. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr. Opin. Cell Biol. 2003, 15, 531–538. [Google Scholar] [CrossRef] [PubMed]
- McEver, R.P. Selectin-carbohydrate interactions during inflammation and metastasis. Glycoconj. J. 1997, 14, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Sulfated glycans in inflammation. Eur. J. Med. Chem. 2015, 92, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, I.R.; Cordeiro, S.L.; Gomes, D.L.; Dreyfuss, J.L.; Filgueira, L.G.; Leite, E.L.; Nader, H.B.; Rocha, H.A. Evaluation of anti-nociceptive and anti-inflammatory activities of a heterofucan from Dictyota menstrualis. Mar. Drugs 2013, 11, 2722–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sousa, A.A.; Benevides, N.M.; de Freitas Pires, A.; Fiúza, F.P.; Queiroz, M.G.; Morais, T.M.; Pereira, M.G.; Assreuy, A.M. A report of a galactan from marine alga Gelidium crinale with in vivo anti-inflammatory and antinociceptive effects. Fundam. Clin. Pharmacol. 2013, 27, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Ye, Q.F. Fucoidan reduces inflammatory response in a rat model of hepatic ischemia-reperfusion injury. Can. J. Physiol. Pharmacol. 2015, 93, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan extracts ameliorate acute colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef] [PubMed]
- Jinno, A.; Park, P.W. Role of glycosaminoglycans in infectious disease. Methods Mol. Biol. 2015, 1229, 567–585. [Google Scholar] [PubMed]
- Sawitzky, D. Protein-glycosaminoglycan interactions: Infectiological aspects. Med. Microbiol. Immunol. 1996, 184, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.J.; Wong, S.L.; Nybakken, K.; Carey, V.J.; Madoff, L.C. Host glycosaminoglycan confers susceptibility to bacterial infection in Drosophila melanogaster. Infect. Immun. 2009, 77, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Wang, Z.; Flax, L.A.; Xu, D.; Esko, J.D.; Nizet, V.; Baron, M.J. Glycosaminoglycan binding facilitates entry of a bacterial pathogen into central nervous systems. PLoS Pathog. 2011, 7, e1002082. [Google Scholar] [CrossRef] [PubMed]
- Sava, I.G.; Zhang, F.; Toma, I.; Theilacker, C.; Li, B.; Baumert, T.F.; Holst, O.; Linhardt, R.J.; Huebner, J. Novel interactions of glycosaminoglycans and bacterial glycolipids mediate binding of enterococci to human cells. J. Biol. Chem. 2009, 284, 18194–18201. [Google Scholar] [CrossRef] [PubMed]
- Wadström, T.; Ljungh, A. Glycosaminoglycan-binding microbial proteins in tissue adhesion and invasion: key events in microbial pathogenicity. J. Med. Microbiol. 1999, 48, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Leistner, C.M.; Gruen-Bernhard, S.; Glebe, D. Role of glycosaminoglycans for binding and infection of hepatitis B virus. Cell. Microbiol. 2008, 10, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Hallak, L.K.; Spillmann, D.; Collins, P.L.; Peeples, M.E. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J. Virol. 2000, 74, 10508–10513. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Banerjee, A.K. Role of heparan sulfate in human parainfluenza virus type 3 infection. Virology 2002, 298, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Vivès, R.R.; Imberty, A.; Sattentau, Q.J.; Lortat-Jacob, H. Heparan sulfate targets the HIV-1 envelope glycoprotein gp120 coreceptor binding site. J. Biol. Chem. 2005, 280, 21353–21357. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Yanagishita, M.; Roderiquez, G.; Bou-Habib, D.C.; Oravecz, T.; Hascall, V.C.; Norcross, M.A. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res. Hum. Retroviruses 1993, 9, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Srinoulprasert, Y.; Kongtawelert, P.; Chaiyaroj, S.C. Chondroitin sulfate B and heparin mediate adhesion of Penicillium marneffei conidia to host extracellular matrices. Microb. Pathog. 2006, 40, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.M.; Reckmann, I.; Hollingdale, M.R.; Bujard, H.; Robson, K.J.; Crisanti, A. Thrombospondin-related anonymous protein (TRAP) of Plasmodium falciparum binds specifically to sulfated glycoconjugates and to HepG2 hepatoma cells, suggesting a role for this molecule in sporozoite invasion of hepatocytes. EMBO J. 1993, 12, 2881–2889. [Google Scholar] [PubMed]
- Yokoyama, N.; Okamura, M.; Igarashi, I. Erythrocyte invasion by Babesia parasites: Current advances in the elucidation of the molecular interactions between the protozoan ligands and host receptors in the invasion stage. Vet. Parasitol. 2006, 138, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Antimicrobial sulfated glycans: Structure and function. Curr. Top. Med. Chem. 2015. [Google Scholar] [CrossRef]
- Rosett, W.; Hodges, G.R. Antimicrobial Activity of Heparin. J. Clin. Microbiol. 1980, 11, 30–34. [Google Scholar] [PubMed]
- Yamada, S.; Sugahara, K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr. Drug Discov. Technol. 2008, 5, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, D.J.; Krylov, V.S. Anti-HIV activity of extracts and compounds from algae and cyanobacteria. Ecotoxicol. Environ. Saf. 2000, 45, 208–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.K. Biological activities of carrageenan. Adv. Food Nutr. Res. 2014, 72, 113–124. [Google Scholar] [PubMed]
- Ahmed, A.B.; Adel, M.; Karimi, P.; Peidayesh, M. Pharmaceutical, cosmeceutical, and traditional applications of marine carbohydrates. Adv. Food Nutr. Res. 2014, 73, 197–220. [Google Scholar] [PubMed]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M. Pilot clinical study to evaluate the anticoagulant activity of fucoidan. Blood Coagul. Fibrinolysis 2009, 20, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.J.; Oliveira, S.N.; Pomin, V.H.; Mecawi, A.S.; Araujo, I.G.; Mourão, P.A. Effects of oversulfated and fucosylated chondroitin sulfates on coagulation. Challenges for the study of anticoagulant polysaccharides. Thromb. Haemost. 2010, 103, 994–1004. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomin, V.H. Marine Non-Glycosaminoglycan Sulfated Glycans as Potential Pharmaceuticals. Pharmaceuticals 2015, 8, 848-864. https://doi.org/10.3390/ph8040848
Pomin VH. Marine Non-Glycosaminoglycan Sulfated Glycans as Potential Pharmaceuticals. Pharmaceuticals. 2015; 8(4):848-864. https://doi.org/10.3390/ph8040848
Chicago/Turabian StylePomin, Vitor H. 2015. "Marine Non-Glycosaminoglycan Sulfated Glycans as Potential Pharmaceuticals" Pharmaceuticals 8, no. 4: 848-864. https://doi.org/10.3390/ph8040848
APA StylePomin, V. H. (2015). Marine Non-Glycosaminoglycan Sulfated Glycans as Potential Pharmaceuticals. Pharmaceuticals, 8(4), 848-864. https://doi.org/10.3390/ph8040848