Changes of Signaling Pathways in Hypothalamic Neurons with Aging
Abstract
:1. Introduction
2. A Brief Description of the Main Hypothalamic Nuclei with a Focus on Mediobasal Hypothalamus
3. Insulin/IGF-1/GH Signaling
4. PI3K/mTOR/AKT Pathway
5. Ras/Raf/MEK/ERK Pathway
6. AMPK Signaling
7. JAK/STAT Signaling
8. Sirtuins
9. Nitric Oxide
10. Hypothalamic Inflammation in Aging
11. Conclusions Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, F.; Zhao, S.; Wang, P.; Xiang, W. Hypothalamic neuroendocrine integration of reproduction and metabolism in mammals. J. Endocrinol. 2023, 258, e230079. [Google Scholar] [CrossRef]
- Haspula, D.; Cui, Z. Neurochemical Basis of Inter-Organ Crosstalk in Health and Obesity: Focus on the Hypothalamus and the Brainstem. Cells 2023, 12, 1801. [Google Scholar] [CrossRef]
- Dilman, V.M. Age-associated elevation of hypothalamic, threshold to feedback control, and its role in development, ageine, and disease. Lancet 1971, 1, 1211–1219. [Google Scholar] [CrossRef]
- Dilman, V.M.; Anisimov, V.N. Hypothalmic mechanisms of ageing and of specific age pathology--I. Sensitivity threshold of hypothalamo-pituitary complex to homeostatic stimuli in the reproductive system. Exp. Gerontol. 1979, 14, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Khor, S. Hypothalamic microinflammation. Handb. Clin. Neurol. 2021, 181, 311–322. [Google Scholar] [CrossRef]
- Kim, K.; Choe, H.K. Role of hypothalamus in aging and its underlying cellular mechanisms. Mech. Ageing Dev. 2019, 177, 74–79. [Google Scholar] [CrossRef]
- Masliukov, P.M.; Nozdrachev, A.D. Hypothalamic Regulatory Mechanisms of Aging. J. Evol. Biochem. Phys. 2021, 57, 473–491. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Purkayastha, S.; Tang, Y.; Zhang, H.; Yin, Y.; Li, B.; Liu, G.; Cai, D. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 2013, 497, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kim, M.S.; Jia, B.; Yan, J.; Zuniga-Hertz, J.P.; Han, C.; Cai, D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 2017, 548, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.A.; Aliper, A.M.; Smit-McBride, Z.; Buzdin, A.; Zhavoronkov, A. Genetics and epigenetics of aging and longevity. Cell Cycle 2014, 13, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, S. Signaling pathways and effectors of aging. Front. Biosci. (Landmark Ed.) 2021, 26, 50–96. [Google Scholar] [CrossRef]
- Qin, C.; Li, J.; Tang, K. The Paraventricular Nucleus of the Hypothalamus: Development, Function, and Human Diseases. Endocrinology 2018, 159, 3458–3472. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.H.; Ludwig, M.; Tasker, J.G.; Stern, J.E. Somato-dendritic vasopressin and oxytocin secretion in endocrine and autonomic regulation. J. Neuroendocrinol. 2020, 32, e12856. [Google Scholar] [CrossRef]
- Wang, L.; Moenter, S.M. Differential Roles of Hypothalamic AVPV and Arcuate Kisspeptin Neurons in Estradiol Feedback Regulation of Female Reproduction. Neuroendocrinology. 2020, 110, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.G.; Kanoski, S.E.; Sanchez-Watts, G.; Langhans, W. The physiological control of eating: Signals, neurons, and networks. Physiol. Rev. 2022, 102, 689–813. [Google Scholar] [CrossRef]
- Patton, A.P.; Hastings, M.H. The suprachiasmatic nucleus. Curr. Biol. 2018, 28, R816–R822. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Nakamura, T.; Yanai, K. Histaminergic neurons in the tuberomammillary nucleus as a control centre for wakefulness. Br. J. Pharmacol. 2021, 178, 750–769. [Google Scholar] [CrossRef]
- Mota, C.M.D.; Madden, C.J. Mediobasal hypothalamic neurons contribute to the control of brown adipose tissue sympathetic nerve activity and cutaneous vasoconstriction. J. Therm. Biol. 2023, 114, 103551. [Google Scholar] [CrossRef]
- Kannangara, H.; Cullen, L.; Miyashita, S.; Korkmaz, F.; Macdonald, A.; Gumerova, A.; Witztum, R.; Moldavski, O.; Sims, S.; Burgess, J.; et al. Emerging roles of brain tanycytes in regulating blood-hypothalamus barrier plasticity and energy homeostasis. Ann. N. Y. Acad. Sci. 2023, 1525, 61–69. [Google Scholar] [CrossRef]
- Vohra, M.S.; Benchoula, K.; Serpell, C.J.; Hwa, W.E. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur. J. Pharmacol. 2022, 915, 174611. [Google Scholar] [CrossRef]
- Decourtye-Espiard, L.; Clemessy, M.; Leneuve, P.; Mire, E.; Ledent, T.; Le Bouc, Y.; Kappeler, L. Stimulation of GHRH Neuron Axon Growth by Leptin and Impact of Nutrition during Suckling in Mice. Nutrients 2023, 15, 1077. [Google Scholar] [CrossRef]
- Vishnyakova, P.A.; Moiseev, K.Y.; Porseval, V.V.; Pankrasheval, L.G.; Budnikl, A.F.; Nozdrachev, A.D.; Masliukov, P.M. Somatostatin-Expressing Neurons in the Tuberal Region of Rat Hypothalamus during Aging. J. Evol. Biochem. Phys. 2021, 57, 1480–1489. [Google Scholar] [CrossRef]
- Prashar, V.; Arora, T.; Singh, R.; Sharma, A.; Parkash, J. Hypothalamic Kisspeptin Neurons: Integral Elements of the GnRH System. Reprod. Sci. 2023, 30, 802–822. [Google Scholar] [CrossRef] [PubMed]
- Velasco, I.; Franssen, D.; Daza-Dueñas, S.; Skrapits, K.; Takács, S.; Torres, E.; Rodríguez-Vazquez, E.; Ruiz-Cruz, M.; León, S.; Kukoricza, K.; et al. Dissecting the KNDy hypothesis: KNDy neuron-derived kisspeptins are dispensable for puberty but essential for preserved female fertility and gonadotropin pulsatility. Metabolism 2023, 144, 155556. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.E.; Coolen, L.M.; Hoffman, G.E.; Hrabovszky, E. Highlights of neuroanatomical discoveries of the mammalian gonadotropin-releasing hormone system. J. Neuroendocrinol. 2022, 34, e13115. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. S1), 5–21. [Google Scholar] [CrossRef]
- Biglari, N.; Gaziano, I.; Schumacher, J.; Radermacher, J.; Paeger, L.; Klemm, P.; Chen, W.; Corneliussen, S.; Wunderlich, C.M.; Sue, M.; et al. Functionally distinct POMC-expressing neuron subpopulations in hypothalamus revealed by intersectional targeting. Nat. Neurosci. 2021, 24, 913–929. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, L.; Zhang, J.; Conde, K.; Phansalkar, J.; Li, Z.; Yao, L.; Xu, Z.; Wang, W.; Zhou, J.; et al. Glucose-sensing glucagon-like peptide-1 receptor neurons in the dorsomedial hypothalamus regulate glucose metabolism. Sci. Adv. 2022, 8, eabn5345. [Google Scholar] [CrossRef]
- Baxter, R.C. Signaling Pathways of the Insulin-like Growth Factor Binding Proteins. Endocr. Rev. 2023, 44, 753–778. [Google Scholar] [CrossRef]
- Chen, W.; Cai, W.; Hoover, B.; Kahn, C.R. Insulin action in the brain: Cell types, circuits, and diseases. Trends Neurosci. 2022, 45, 384–400. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, X.; Batista, T.M.; García-Martín, R.; Softic, S.; Wang, G.; Ramirez, A.K.; Konishi, M.; O’Neill, B.T.; Kim, J.H.; et al. Peripheral Insulin Regulates a Broad Network of Gene Expression in Hypothalamus, Hippocampus, and Nucleus Accumbens. Diabetes 2021, 70, 1857–1873. [Google Scholar] [CrossRef]
- Hakuno, F.; Takahashi, S.I. IGF1 receptor signaling pathways. J. Mol. Endocrinol. 2018, 61, T69–T86. [Google Scholar] [CrossRef]
- Jiráček, J.; Selicharová, I.; Žáková, L. Mutations at hypothetical binding site 2 in insulin and insulin-like growth factors 1 and 2. Vitam. Horm. 2023, 123, 187–230. [Google Scholar] [CrossRef]
- Pomytkin, I.; Costa-Nunes, J.P.; Kasatkin, V.; Veniaminova, E.; Demchenko, A.; Lyundup, A.; Lesch, K.P.; Ponomarev, E.D.; Strekalova, T. Insulin receptor in the brain: Mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci. Ther. 2018, 24, 763–774. [Google Scholar] [CrossRef]
- Li, M.; Quan, C.; Toth, R.; Campbell, D.G.; MacKintosh, C.; Wang, H.Y.; Chen, S. Fasting and Systemic Insulin Signaling Regulate Phosphorylation of Brain Proteins That Modulate Cell Morphology and Link to Neurological Disorders. J. Biol. Chem. 2015, 290, 30030–30041. [Google Scholar] [CrossRef]
- Rotwein, P. Regulation of gene expression by growth hormone. Mol. Cell Endocrinol. 2020, 507, 110788. [Google Scholar] [CrossRef] [PubMed]
- Al-Samerria, S.; Radovick, S. Exploring the Therapeutic Potential of Targeting GH and IGF-1 in the Management of Obesity: Insights from the Interplay between These Hormones and Metabolism. Int. J. Mol. Sci. 2023, 24, 9556. [Google Scholar] [CrossRef]
- Al-Samerria, S.; Radovick, S. The Role of Insulin-like Growth Factor-1 (IGF-1) in the Control of Neuroendocrine Regulation of Growth. Cells 2021, 10, 2664. [Google Scholar] [CrossRef]
- Likitnukul, S.; Thammacharoen, S.; Sriwatananukulkit, O.; Duangtha, C.; Hemstapat, R.; Sunrat, C.; Mangmool, S.; Pinthong, D. Short-Term Growth Hormone Administration Mediates Hepatic Fatty Acid Uptake and De Novo Lipogenesis Gene Expression in Obese Rats. Biomedicines 2023, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Høgild, M.L.; Hjelholt, A.J.; Hansen, J.; Pedersen, S.B.; Møller, N.; Wojtaszewski, J.F.P.; Johannsen, M.; Jessen, N.; Jørgensen, J.O.L. Ketone Body Infusion Abrogates Growth Hormone-Induced Lipolysis and Insulin Resistance. J. Clin. Endocrinol. Metab. 2023, 108, 653–664. [Google Scholar] [CrossRef]
- Sharma, R.; Kopchick, J.J.; Puri, V.; Sharma, V.M. Effect of growth hormone on insulin signaling. Mol. Cell Endocrinol. 2020, 518, 111038. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Szablewski, L. Insulin and Insulin Resistance in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Becattini, B. PI3K and AKT at the Interface of Signaling and Metabolism. Curr. Top Microbiol. Immunol. 2022, 436, 311–336. [Google Scholar] [CrossRef]
- Kumar, M.; Bansal, N. Implications of Phosphoinositide 3-Kinase-Akt (PI3K-Akt) Pathway in the Pathogenesis of Alzheimer’s Disease. Mol. Neurobiol. 2022, 59, 354–385. [Google Scholar] [CrossRef]
- Varela, L.; Horvath, T.L. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012, 13, 1079–1086. [Google Scholar] [CrossRef]
- Bergan-Roller, H.E.; Sheridan, M.A. The growth hormone signaling system: Insights into coordinating the anabolic and catabolic actions of growth hormone. Gen. Comp. Endocrinol. 2018, 258, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Ashpole, N.M.; Sanders, J.E.; Hodges, E.L.; Yan, H.; Sonntag, W.E. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp. Gerontol. 2015, 68, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Spik, K.; Sonntag, W.E. Increased pituitary response to somatostatin in aging male rats: Relationship to somatostatin receptor number and affinity. Neuroendocrinology 1989, 50, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Biagetti, B.; Puig-Domingo, M. Age-Related Hormones Changes and Its Impact on Health Status and Lifespan. Aging Dis. 2023, 14, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.; Salvatori, R. Growth Hormone and Aging. Endocrinol. Metab. Clin N. Am. 2023, 52, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A. Growth Hormone and Aging: Updated Review. World J. Mens. Health 2019, 37, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Dravecz, N.; Shaw, T.; Davies, I.; Brown, C.; Ormerod, L.; Vu, G.; Walker, T.; Taank, T.; Shirras, A.D.; Broughton, S.J. Reduced Insulin Signaling Targeted to Serotonergic Neurons but Not Other Neuronal Subtypes Extends Lifespan in Drosophila melanogaster. Front. Aging Neurosci. 2022, 14, 893444. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.V. Recent Progress in Regulation of Aging by Insulin/IGF-1 Signaling in Caenorhabditis elegans. Mol. Cells 2022, 45, 763–770. [Google Scholar] [CrossRef]
- Bartke, A.; Brown-Borg, H. Mutations Affecting Mammalian Aging: GH and GHR vs IGF-1 and Insulin. Front. Genet. 2021, 12, 667355. [Google Scholar] [CrossRef]
- Liu, J.P.; Baker, J.; Perkins, A.S.; Robertson, E.J.; Efstratiadis, A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 1993, 75, 59–72. [Google Scholar] [CrossRef]
- Accili, D.; Drago, J.; Lee, E.J.; Johnson, M.D.; Cool, M.H.; Salvatore, P.; Asico, L.D.; José, P.A.; Taylor, S.I.; Westphal, H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat. Genet. 1996, 12, 106–109. [Google Scholar] [CrossRef]
- Rincon, M.; Muzumdar, R.; Atzmon, G.; Barzilai, N. The paradox of the insulin/IGF-1 signaling pathway in longevity. Mech. Ageing Dev. 2004, 125, 397–403. [Google Scholar] [CrossRef]
- Bokov, A.F.; Garg, N.; Ikeno, Y.; Thakur, S.; Musi, N.; DeFronzo, R.A.; Zhang, N.; Erickson, R.C.; Gelfond, J.; Hubbard, G.B.; et al. Does reduced IGF-1R signaling in Igf1r+/− mice alter aging? PLoS ONE 2011, 6, e26891. [Google Scholar] [CrossRef]
- Agrawal, R.; Reno, C.M.; Sharma, S.; Christensen, C.; Huang, Y.; Fisher, S.J. Insulin action in the brain regulates both central and peripheral functions. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E156–E163. [Google Scholar] [CrossRef]
- Ezkurdia, A.; Ramírez, M.J.; Solas, M. Metabolic Syndrome as a Risk Factor for Alzheimer’s Disease: A Focus on Insulin Resistance. Int. J. Mol. Sci. 2023, 24, 4354. [Google Scholar] [CrossRef]
- Shpakov, A.O.; Derkach, K.V.; Berstein, L.M. Brain signaling systems in the Type 2 diabetes and metabolic syndrome: Promising target to treat and prevent these diseases. Future Sci. OA 2015, 1, FSO25. [Google Scholar] [CrossRef]
- Merry, T.L.; Kuhlow, D.; Laube, B.; Pöhlmann, D.; Pfeiffer, A.F.H.; Kahn, C.R.; Ristow, M.; Zarse, K. Impairment of insulin signalling in peripheral tissue fails to extend murine lifespan. Aging Cell 2017, 16, 761–772. [Google Scholar] [CrossRef]
- Blüher, M.; Kahn, B.B.; Kahn, C.R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 2003, 299, 572–574. [Google Scholar] [CrossRef]
- Ono, H. Molecular Mechanisms of Hypothalamic Insulin Resistance. Int. J. Mol. Sci. 2019, 20, 1317. [Google Scholar] [CrossRef]
- Könner, A.C.; Janoschek, R.; Plum, L.; Jordan, S.D.; Rother, E.; Ma, X.; Xu, C.; Enriori, P.; Hampel, B.; Barsh, G.S.; et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007, 5, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.C.; Filatova, N.; Lindtner, C.; Chi, T.; Degann, S.; Oberlin, D.; Buettner, C. Insulin Receptor Signaling in POMC, but Not AgRP, Neurons Controls Adipose Tissue Insulin Action. Diabetes 2017, 66, 1560–1571. [Google Scholar] [CrossRef]
- Hausen, A.C.; Ruud, J.; Jiang, H.; Hess, S.; Varbanov, H.; Kloppenburg, P.; Brüning, J.C. Insulin-Dependent Activation of MCH Neurons Impairs Locomotor Activity and Insulin Sensitivity in Obesity. Cell Rep. 2016, 17, 2512–2521. [Google Scholar] [CrossRef]
- Bhalla, S.; Mehan, S.; Khan, A.; Rehman, M.U. Protective role of IGF-1 and GLP-1 signaling activation in neurological dysfunctions. Neurosci. Biobehav. Rev. 2022, 142, 104896. [Google Scholar] [CrossRef]
- Shandilya, A.; Mehan, S. Dysregulation of IGF-1/GLP-1 signaling in the progression of ALS: Potential target activators and influences on neurological dysfunctions. Neurol. Sci. 2021, 42, 3145–3166. [Google Scholar] [CrossRef]
- Cignarelli, A.; Genchi, V.A.; Le Grazie, G.; Caruso, I.; Marrano, N.; Biondi, G.; D’Oria, R.; Sorice, G.P.; Natalicchio, A.; Perrini, S.; et al. Mini Review: Effect of GLP-1 Receptor Agonists and SGLT-2 Inhibitors on the Growth Hormone/IGF Axis. Front. Endocrinol. 2022, 13, 846903. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Cheng, L.; Li, J.; Zhang, T.; Zhao, G.; Zhang, H. Glucagon-Like Peptide-1 Cleavage Product GLP-1(9–36) Reduces Neuroinflammation From Stroke via the Activation of Insulin-Like Growth Factor 1 Receptor in Astrocytes. Eur. J. Pharmacol. 2020, 887, 173581. [Google Scholar] [CrossRef]
- Okada, T.; Mita, Y.; Sakoda, H.; Nakazato, M. Impaired adaptation of energy intake induces severe obesity in aged mice on a high-fat diet. Physiol. Rep. 2019, 7, e13989. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.C.; McKern, N.M.; Ward, C.W. Insulin receptor structure and its implications for the IGF-1 receptor. Curr. Opin. Struct. Biol. 2007, 17, 699–705. [Google Scholar] [CrossRef]
- Björnholm, M.; He, A.R.; Attersand, A.; Lake, S.; Liu, S.C.; Lienhard, G.E.; Taylor, S.; Arner, P.; Zierath, J.R. Absence of functional insulin receptor substrate-3 (IRS-3) gene in humans. Diabetologia 2002, 45, 1697–1702. [Google Scholar] [CrossRef]
- Ling, Y.; Maile, L.A.; Badley-Clarke, J.; Clemmons, D.R. DOK1 mediates SHP-2 binding to the alphaVbeta3 integrin and thereby regulates insulin-like growth factor I signaling in cultured vascular smooth muscle cells. J. Biol. Chem. 2005, 280, 3151–3158. [Google Scholar] [CrossRef]
- Safaroghli-Azar, A.; Sanaei, M.J.; Pourbagheri-Sigaroodi, A.; Bashash, D. Phosphoinositide 3-kinase (PI3K) classes: From cell signaling to endocytic recycling and autophagy. Eur. J. Pharmacol. 2023, 953, 175827. [Google Scholar] [CrossRef]
- Medina-Vera, D.; Navarro, J.A.; Tovar, R.; Rosell-Valle, C.; Gutiérrez-Adan, A.; Ledesma, J.C.; Sanjuan, C.; Pavón, F.J.; Baixeras, E.; Rodríguez de Fonseca, F.; et al. Activation of PI3K/Akt Signaling Pathway in Rat Hypothalamus Induced by an Acute Oral Administration of D-Pinitol. Nutrients 2021, 13, 2268. [Google Scholar] [CrossRef]
- Dibble, C.C.; Cantley, L.C. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015, 25, 545–555. [Google Scholar] [CrossRef]
- Saltiel, A.R. Insulin signaling in health and disease. J Clin Investig. 2021, 131, e142241. [Google Scholar] [CrossRef]
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018, 19, 31–44. [Google Scholar] [CrossRef]
- Simcox, J.; Lamming, D.W. The central moTOR of metabolism. Dev. Cell. 2022, 57, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Hakuno, F.; Fukushima, T.; Yoneyama, Y.; Kamei, H.; Ozoe, A.; Yoshihara, H.; Yamanaka, D.; Shibano, T.; Sone-Yonezawa, M.; Yu, B.C.; et al. The Novel Functions of High-Molecular-Mass Complexes Containing Insulin Receptor Substrates in Mediation and Modulation of Insulin-Like Activities: Emerging Concept of Diverse Functions by IRS-Associated Proteins. Front. Endocrinol. 2015, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Yoshihara, H.; Furuta, H.; Kamei, H.; Hakuno, F.; Luan, J.; Duan, C.; Saeki, Y.; Tanaka, K.; Iemura, S.; et al. Nedd4-induced monoubiquitination of IRS-2 enhances IGF signalling and mitogenic activity. Nat. Commun. 2015, 6, 6780. [Google Scholar] [CrossRef]
- Selman, C.; Lingard, S.; Choudhury, A.I.; Batterham, R.L.; Claret, M.; Clements, M.; Ramadani, F.; Okkenhaug, K.; Schuster, E.; Blanc, E.; et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008, 22, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, M.; Nespital, T.; Mesaros, A.; Buschbaum, S.; Withers, D.J.; Grönke, S.; Partridge, L. Reduced insulin signaling in neurons induces sex-specific health benefits. Sci. Adv. 2023, 9, eade8137. [Google Scholar] [CrossRef]
- Taguchi, A.; Wartschow, L.M.; White, M.F. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 2007, 317, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; Lingard, S.; Gems, D.; Partridge, L.; Withers, D.J. Comment on “Brain IRS2 signaling coordinates life span and nutrient homeostasis”. Science 2008, 320, 1012. [Google Scholar] [CrossRef]
- Valverde, A.M.; Mur, C.; Pons, S.; Alvarez, A.M.; White, M.F.; Kahn, C.R.; Benito, M. Association of insulin receptor substrate 1 (IRS-1) y895 with Grb-2 mediates the insulin signaling involved in IRS-1-deficient brown adipocyte mitogenesis. Mol. Cell Biol. 2001, 21, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Russo, B.; Menduni, M.; Borboni, P.; Picconi, F.; Frontoni, S. Autonomic Nervous System in Obesity and Insulin-Resistance-The Complex Interplay between Leptin and Central Nervous System. Int. J. Mol. Sci. 2021, 22, 5187. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling pathways in obesity: Mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Dysregulation of PI3K-Akt-mTOR pathway in brain of streptozotocin-induced type 2 diabetes mellitus in Wistar rats. Lipids Health Dis. 2018, 17, 168. [Google Scholar] [CrossRef]
- Ma, Y.; Murgia, N.; Liu, Y.; Li, Z.; Sirakawin, C.; Konovalov, R.; Kovzel, N.; Xu, Y.; Kang, X.; Tiwari, A.; et al. Neuronal miR-29a protects from obesity in adult mice. Mol. Metab. 2022, 61, 101507. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Kim, K.W.; Kim, M.S. Leptin signalling pathways in hypothalamic neurons. Cell. Mol. Life Sci. 2016, 73, 1457–1477. [Google Scholar] [CrossRef] [PubMed]
- Benite-Ribeiro, S.A.; Rodrigues, V.A.L.; Machado, M.R.F. Food intake in early life and epigenetic modifications of pro-opiomelanocortin expression in arcuate nucleus. Mol. Biol. Rep. 2021, 48, 3773–3784. [Google Scholar] [CrossRef]
- Williams, K.W.; Margatho, L.O.; Lee, C.E.; Choi, M.; Lee, S.; Scott, M.M.; Elias, C.F.; Elmquist, J.K. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J. Neurosci. 2010, 30, 2472–2479. [Google Scholar] [CrossRef]
- Borges, B.C.; Elias, C.F.; Elias, L.L. PI3K signaling: A molecular pathway associated with acute hypophagic response during inflammatory challenges. Mol. Cell Endocrinol. 2016, 438, 36–41. [Google Scholar] [CrossRef]
- Bharill, P.; Ayyadevara, S.; Alla, R.; Shmookler Reis, R.J. Extreme Depletion of PIP3 Accompanies the Increased Life Span and Stress Tolerance of PI3K-null C. elegans Mutants. Front. Genet. 2013, 4, 34. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Revathidevi, S.; Munirajan, A.K. Akt in cancer: Mediator and more. Semin. Cancer Biol. 2019, 59, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Kim, D.H.; Lee, E.K.; Kim, N.D.; Im, D.S.; Lee, J.; Yu, B.P.; Chung, H.Y. Age-related inflammation and insulin resistance: A review of their intricate interdependency. Arch. Pharm. Res. 2014, 37, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, B.; Fei, J.; Santanam, N.; Blough, E.R. Important roles of Akt/PKB signaling in the aging process. Front. Biosci. (Schol. Ed.) 2010, 2, 1169–1188. [Google Scholar] [CrossRef]
- Kim, D.H.; Bang, E.; Ha, S.; Jung, H.J.; Choi, Y.J.; Yu, B.P.; Chung, H.Y. Organ-differential Roles of Akt/FoxOs Axis as a Key Metabolic Modulator during Aging. Aging Dis. 2021, 12, 1713–1728. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tao, X.; Sun, R.; Han, C.; Li, X.; Zhu, Z.; Li, W.; Huang, P.; Gong, W. Cognitive-exercise dual-task intervention ameliorates cognitive decline in natural aging rats via inhibiting the promotion of LncRNA NEAT1/miR-124-3p on caveolin-1-PI3K/Akt/GSK3β Pathway. Brain Res. Bull. 2023, 202, 110761. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xuanyuan, S.; Zhang, B.; Shi, C. Activation of PI3K/Akt prevents hypoxia/reoxygenation-induced GnRH decline via FOXO3a. Physiol. Res. 2022, 71, 509–516. [Google Scholar] [CrossRef]
- Yang, S.; Du, Y.; Zhao, X.; Wu, C.; Yu, P. Reducing PDK1/Akt Activity: An Effective Therapeutic Target in the Treatment of Alzheimer’s Disease. Cells 2022, 11, 1735. [Google Scholar] [CrossRef]
- Chen, Y.R.; Li, Y.H.; Hsieh, T.C.; Wang, C.M.; Cheng, K.C.; Wang, L.; Lin, T.Y.; Cheung, C.H.A.; Wu, C.L.; Chiang, H. Aging-induced Akt activation involves in aging-related pathologies and Aβ-induced toxicity. Aging Cell 2019, 18, e12989. [Google Scholar] [CrossRef] [PubMed]
- García-San Frutos, M.; Fernández-Agulló, T.; De Solís, A.J.; Andrés, A.; Arribas, C.; Carrascosa, J.M.; Ros, M. Impaired central insulin response in aged Wistar rats: Role of adiposity. Endocrinology 2007, 148, 5238–5247. [Google Scholar] [CrossRef]
- Anfimova, P.A.; Pankrasheva, L.G.; Moiseev, K.Y.; Shirina, E.S.; Porseva, V.V.; Masliukov, P.M. Ontogenetic Changes in the Expression of the Lin28 Protein in the Rat Hypothalamic Tuberal Nuclei. Int. J. Mol. Sci. 2022, 23, 13468. [Google Scholar] [CrossRef]
- Du, S.; Zheng, H. Role of FoxO transcription factors in aging and age-related metabolic and neurodegenerative diseases. Cell Biosci. 2021, 11, 188. [Google Scholar] [CrossRef]
- Shi, C.; Shi, R.; Guo, H. Tumor necrosis factor α reduces gonadotropin-releasing hormone release through increase of forkhead box protein O1 activity. Neuroreport 2020, 31, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, H. Modulation of Sirt1 and FoxO1 on Hypothalamic Leptin-Mediated Sympathetic Activation and Inflammation in Diet-Induced Obese Rats. J. Am. Heart Assoc. 2021, 10, e020667. [Google Scholar] [CrossRef] [PubMed]
- Dakic, T.; Jevdjovic, T.; Djordjevic, J.; Vujovic, P. Short-term fasting differentially regulates PI3K/AkT/mTOR and ERK signalling in the rat hypothalamus. Mech. Ageing Dev. 2020, 192, 111358. [Google Scholar] [CrossRef] [PubMed]
- Papadopoli, D.; Boulay, K.; Kazak, L.; Pollak, M.; Mallette, F.; Topisirovic, I.; Hulea, L. mTOR as a central regulator of lifespan and aging. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Chrienova, Z.; Nepovimova, E.; Kuca, K. The role of mTOR in age-related diseases. J. Enzyme Inhib. Med. Chem. 2021, 36, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, L.; Qin, S.; Zhang, T.; Yao, J.; Yi, Y.; Deng, L. Mechanistic Target of Rapamycin Complex 1: From a Nutrient Sensor to a Key Regulator of Metabolism and Health. Adv. Nutr. 2022, 13, 1882–1900. [Google Scholar] [CrossRef] [PubMed]
- Saoudaoui, S.; Bernard, M.; Cardin, G.B.; Malaquin, N.; Christopoulos, A.; Rodier, F. mTOR as a senescence manipulation target: A forked road. Adv. Cancer Res. 2021, 150, 335–363. [Google Scholar] [CrossRef]
- Muta, K.; Morgan, D.A.; Rahmouni, K. The role of hypothalamic mTORC1 signaling in insulin regulation of food intake, body weight, and sympathetic nerve activity in male mice. Endocrinology 2015, 156, 1398–1407. [Google Scholar] [CrossRef]
- Caron, A.; Labbé, S.M.; Lanfray, D.; Blanchard, P.G.; Villot, R.; Roy, C.; Sabatini, D.M.; Richard, D.; Laplante, M. Mediobasal hypothalamic overexpression of DEPTOR protects against high-fat diet-induced obesity. Mol. Metab. 2015, 5, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Anfimova, P.A.; Moiseev, K.Y.; Porseva, V.V.; Pankrasheva, L.G.; Masliukov, P.M. mTOR Expression in Neurons of the Rat Tuberal Hypothalamus in Aging. J. Evol. Biochem. Phys. 2022, 58, 1464–1470. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct Target Ther. 2023, 8, 375. [Google Scholar] [CrossRef]
- Chellappa, K.; Brinkman, J.A.; Mukherjee, S.; Morrison, M.; Alotaibi, M.I.; Carbajal, K.A.; Alhadeff, A.L.; Perron, I.J.; Yao, R.; Purdy, C.S.; et al. Hypothalamic mTORC2 is essential for metabolic health and longevity. Aging Cell 2019, 18, e13014. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Insulin/IGF-1 signaling promotes immunosuppression via the STAT3 pathway: Impact on the aging process and age-related diseases. Inflamm. Res. 2021, 70, 1043–1061. [Google Scholar] [CrossRef]
- Yoshizawa, R.; Umeki, N.; Yamamoto, A.; Murata, M.; Sako, Y. Biphasic spatiotemporal regulation of GRB2 dynamics by p52SHC for transient RAS activation. Biophys. Physicobiol. 2021, 18, 1–12. [Google Scholar] [CrossRef]
- Ullah, R.; Yin, Q.; Snell, A.H.; Wan, L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin. Cancer Biol. 2022, 85, 123–154. [Google Scholar] [CrossRef]
- Milstein, J.L.; Ferris, H.A. The brain as an insulin-sensitive metabolic organ. Mol. Metab. 2021, 52, 101234. [Google Scholar] [CrossRef]
- Kleinridders, A. Deciphering Brain Insulin Receptor and Insulin-Like Growth Factor 1 Receptor Signalling. J. Neuroendocrinol. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; O’Hare, J.; Cheng, B.; Puchowicz, M.; Buettner, C.; Scherer, T. Brain insulin signaling suppresses lipolysis in the absence of peripheral insulin receptors and requires the MAPK pathway. Mol. Metab. 2023, 73, 101723. [Google Scholar] [CrossRef]
- Karmarkar, S.W.; Bottum, K.M.; Krager, S.L.; Tischkau, S.A. ERK/MAPK is essential for endogenous neuroprotection in SCN2.2 cells. PLoS ONE 2011, 6, e23493. [Google Scholar] [CrossRef]
- Maher, P.; Dargusch, R.; Bodai, L.; Gerard, P.E.; Purcell, J.M.; Marsh, J.L. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington’s disease. Hum. Mol. Genet. 2011, 20, 261–270. [Google Scholar] [CrossRef]
- Zhen, X.; Uryu, K.; Cai, G.; Johnson, G.P.; Friedman, E. Age-associated impairment in brain MAPK signal pathways and the effect of caloric restriction in Fischer 344 rats. J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, B539–B548. [Google Scholar] [CrossRef]
- Song, G.Y.; Kang, J.S.; Lee, S.Y.; Myung, C.S. Region-specific reduction of Gbeta4 expression and induction of the phosphorylation of PKB/Akt and ERK1/2 by aging in rat brain. Pharmacol. Res. 2007, 56, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.M.; Belsham, D.D. Insulin directly regulates NPY and AgRP gene expression via the MAPK MEK/ERK signal transduction pathway in mHypoE-46 hypothalamic neurons. Mol. Cell Endocrinol. 2009, 307, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, R.; Gabriele, C.; Santamaria, G.; Giuliano, M.; Vella, V.; Massimino, M.; Vigneri, P.; Cuda, G.; Gaspari, M.; Belfiore, A. Comparative proteomic analysis of insulin receptor isoform A and B signaling. Mol Cell Endocrinol. 2022, 557, 111739. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.A.; Muñoz, V.R.; Kuga, G.K.; Gaspar, R.C.; Nakandakari, S.C.B.R.; Crisol, B.M.; Botezelli, J.D.; Pauli, L.S.S.; da Silva, A.S.R.; de Moura, L.P.; et al. Obesity Increases Mitogen-Activated Protein Kinase Phosphatase-3 Levels in the Hypothalamus of Mice. Front. Cell Neurosci. 2017, 11, 313. [Google Scholar] [CrossRef]
- Obrietan, K.; Impey, S.; Storm, D.R. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat. Neurosci. 1998, 1, 693–700. [Google Scholar] [CrossRef]
- Alzate-Correa, D.; Aten, S.; Campbell, M.J.; Hoyt, K.R.; Obrietan, K. Light-induced changes in the suprachiasmatic nucleus transcriptome regulated by the ERK/MAPK pathway. PLoS ONE 2021, 16, e0249430. [Google Scholar] [CrossRef]
- Sharma, A.; Anandl, S.K.; Singh, N.; Dwivedi, U.N.; Kakkar, P. AMP-activated protein kinase: An energy sensor and survival mechanism in the reinstatement of metabolic homeostasis. Exp. Cell Res. 2023, 428, 113614. [Google Scholar] [CrossRef]
- Saikia, R.; Joseph, J. AMPK: A key regulator of energy stress and calcium-induced autophagy. J. Mol. Med. 2021, 99, 1539–1551. [Google Scholar] [CrossRef]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhou, M.; Chen, C.; Wu, X.; Wang, X. Role of AMPK mediated pathways in autophagy and aging. Biochimie 2022, 195, 100–113. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Age-related changes in AMPK activation: Role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Res. Rev. 2016, 28, 15–26. [Google Scholar] [CrossRef]
- Ning, J.; Xi, G.; Clemmons, D.R. Suppression of AMPK activation via S485 phosphorylation by IGF-I during hyperglycemia is mediated by AKT activation in vascular smooth muscle cells. Endocrinology 2011, 152, 3143–3154. [Google Scholar] [CrossRef]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. PI3K/AKT, MAPK and AMPK signalling: Protein kinases in glucose homeostasis. Exp. Rev. Mol. Med. 2012, 14, e1. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.D.S.; Oliveira, R.K.G.; Dos Santos, L.S.; Ribeiro, I.O.; Barreto-Medeiros, J.M.B.; Matos, R.J.B. Modulation of hypothalamic AMPK and hypothalamic neuropeptides in the control of eating behavior: A systematic review. Life Sci. 2022, 309, 120947. [Google Scholar] [CrossRef]
- Song, K.; Zhang, Y.; Ga, Q.; Bai, Z.; Ge, R.L. Increased Insulin Sensitivity by High-Altitude Hypoxia in Mice with High-Fat Diet-Induced Obesity Is Associated with Activated AMPK Signaling and Subsequently Enhanced Mitochondrial Biogenesis in Skeletal Muscles. Obes. Facts 2020, 13, 455–472. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Alquier, T.; Furukawa, N.; Kim, Y.B.; Lee, A.; Xue, B.; Mu, J.; Foufelle, F.; Ferré, P.; Birnbaum, M.J.; et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004, 428, 569–574. [Google Scholar] [CrossRef]
- Claret, M.; Smith, M.A.; Batterham, R.L.; Selman, C.; Choudhury, A.I.; Fryer, L.G.; Clements, M.; Al-Qassab, H.; Heffron, H.; Xu, A.W.; et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J. Clin. Investig. 2007, 117, 2325–2336. [Google Scholar] [CrossRef]
- López, M.; Lage, R.; Saha, A.K.; Pérez-Tilve, D.; Vázquez, M.J.; Varela, L.; Sangiao-Alvarellos, S.; Tovar, S.; Raghay, K.; Rodríguez-Cuenca, S.; et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008, 7, 389–399. [Google Scholar] [CrossRef]
- Cavaliere, G.; Viggiano, E.; Trinchese, G.; De Filippo, C.; Messina, A.; Monda, V.; Valenzano, A.; Cincione, R.I.; Zammit, C.; Cimmino, F.; et al. Long Feeding High-Fat Diet Induces Hypothalamic Oxidative Stress and Inflammation, and Prolonged Hypothalamic AMPK Activation in Rat Animal Model. Front. Physiol. 2018, 9, 818. [Google Scholar] [CrossRef]
- Toklu, H.Z.; Scarpace, P.J.; Sakarya, Y.; Kirichenko, N.; Matheny, M.; Bruce, E.B.; Carter, C.S.; Morgan, D.; Tümer, N. Intracerebroventricular tempol administration in older rats reduces oxidative stress in the hypothalamus but does not change STAT3 signalling or SIRT1/AMPK pathway. Appl. Physiol. Nutr. Metab. 2017, 42, 59–67. [Google Scholar] [CrossRef]
- Xu, W.; Luo, Y.; Yin, J.; Huang, M.; Luo, F. Targeting AMPK signaling by polyphenols: A novel strategy for tackling aging. Food Funct. 2023, 14, 56–73. [Google Scholar] [CrossRef]
- López, M. Hypothalamic AMPK as a possible target for energy balance-related diseases. Trends Pharmacol. Sci. 2022, 43, 546–556. [Google Scholar] [CrossRef]
- Chau-Van, C.; Gamba, M.; Salvi, R.; Gaillard, R.C.; Pralong, F.P. Metformin inhibits adenosine 5’-monophosphate-activated kinase activation and prevents increases in neuropeptide Y expression in cultured hypothalamic neurons. Endocrinology 2007, 148, 507–511. [Google Scholar] [CrossRef]
- Derkach, K.; Zakharova, I.; Zorina, I.; Bakhtyukov, A.; Romanova, I.; Bayunova, L.; Shpakov, A. The evidence of metabolic-improving effect of metformin in Ay/a mice with genetically-induced melanocortin obesity and the contribution of hypothalamic mechanisms to this effect. PLoS ONE 2019, 14, e0213779. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef]
- Dodington, D.W.; Desai, H.R.; Woo, M. JAK/STAT—Emerging Players in Metabolism. Trends Endocrinol. Metab. 2018, 29, 55–65. [Google Scholar] [CrossRef]
- Villarino, A.V.; Kanno, Y.; Ferdinand, J.R.; O’Shea, J.J. Mechanisms of Jak/STAT signaling in immunity and disease. J. Immunol. 2015, 194, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, T.; Li, C.; Yang, G. STAT3 phosphorylation in central leptin resistance. Nutr. Metab. 2021, 18, 39. [Google Scholar] [CrossRef]
- Strous, G.J.; Almeida, A.D.S.; Putters, J.; Schantl, J.; Sedek, M.; Slotman, J.A.; Nespital, T.; Hassink, G.C.; Mol, J.A. Growth Hormone Receptor Regulation in Cancer and Chronic Diseases. Front. Endocrinol. 2020, 11, 597573. [Google Scholar] [CrossRef]
- Zieba, D.A.; Biernat, W.; Barć, J. Roles of leptin and resistin in metabolism, reproduction, and leptin resistance. Domest. Anim. Endocrinol. 2020, 106472. [Google Scholar] [CrossRef] [PubMed]
- Scarpace, P.J.; Matheny, M.; Tümer, N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience 2001, 104, 1111–1117. [Google Scholar] [CrossRef]
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chojdak-Łukasiewicz, J.; Bizoń, A.; Waliszewska-Prosół, M.; Piwowar, A.; Budrewicz, S.; Pokryszko-Dragan, A. Role of Sirtuins in Physiology and Diseases of the Central Nervous System. Biomedicines 2022, 10, 2434. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid Redox Signal 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Lu, C.; Zhao, H.; Liu, Y.; Yang, Z.; Yao, H.; Liu, T.; Gou, T.; Wang, L.; Zhang, J.; Tian, Y.; et al. Novel Role of the SIRT1 in Endocrine and Metabolic Diseases. Int. J. Biol. Sci. 2023, 19, 484–501. [Google Scholar] [CrossRef] [PubMed]
- Ungurianu, A.; Zanfirescu, A.; Margină, D. Sirtuins, resveratrol and the intertwining cellular pathways connecting them. Ageing Res. Rev. 2023, 88, 101936. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct Target Ther. 2022, 7, 402. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Pukhalskaia, A.E.; Kvetnoy, I.M.; Linkova, N.S.; Diatlova, A.S.; Gutop, E.O.; Kozlov, K.L.; Paltsev, M.A. Sirtuins and Aging. Neurosci. Behav. Physiol. 2022, 52, 1482–1490. [Google Scholar] [CrossRef]
- Satoh, A.; Imaim, S.I.; Guarente, L. The brain, sirtuins, and ageing. Nat. Rev. Neurosci. 2017, 18, 362–374. [Google Scholar] [CrossRef]
- Mayor, E. Neurotrophic effects of intermittent fasting, calorie restriction and exercise: A review and annotated bibliography. Front. Aging 2023, 4, 1161814. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Misrani, A.; Huang, H.X.; Zhang, Z.Y.; Li, Q.W.; Long, C. Resveratrol Attenuates Chronic Unpredictable Mild Stress-Induced Alterations in the SIRT1/PGC1α/SIRT3 Pathway and Associated Mitochondrial Dysfunction in Mice. Mol. Neurobiol. 2023, 60, 5102–5116. [Google Scholar] [CrossRef] [PubMed]
- Spirichev, A.A.; Moiseev, K.Y.; Masliukov, P.M. Sirtuin 1 Expression in the Rat Ventromedial and Dorsomedial Hypothalamic Nuclei during Ageing. Bull. Exp. Biol. Med. 2020, 169, 698–700. [Google Scholar] [CrossRef]
- Kukkemane, K.; Jagota, A. Therapeutic effects of hydro-alcoholic leaf extract of Withania somnifera on age-induced changes in daily rhythms of Sirt1, Nrf2 and Rev-erbα in the SCN of male Wistar rats. Biogerontology 2020, 21, 593–607. [Google Scholar] [CrossRef]
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef]
- Sasaki, T.; Kitamura, T. Roles of FoxO1 and Sirt1 in the central regulation of food intake. Endocr. J. 2010, 57, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Carlström, M.; Mirmiran, P.; Ghasemi, A. Nitric oxide: To be or not to be an endocrine hormone? Acta Physiol. 2020, 229, e13443. [Google Scholar] [CrossRef] [PubMed]
- Chachlaki, K.; Prevot, V. Nitric oxide signalling in the brain and its control of bodily functions. Br. J. Pharmacol. 2020, 177, 5437–5458. [Google Scholar] [CrossRef]
- Chachlaki, K.; Garthwaite, J.; Prevot, V. The gentle art of saying NO: How nitric oxide gets things done in the hypothalamus. Nat. Rev. Endocrinol. 2017, 9, 521–535. [Google Scholar] [CrossRef]
- Delli, V.; Silva, M.S.B.; Prévot, V.; Chachlaki, K. The KiNG of reproduction: Kisspeptin/nNOS interactions shaping hypothalamic GnRH release. Mol. Cell. Endocrinol. 2021, 532, 111302. [Google Scholar] [CrossRef] [PubMed]
- Chachlaki, K.; Malone, S.A.; Qualls-Creekmore, E.; Hrabovszky, E.; Münzberg, H.; Giacobini, P.; Ango, F.; Prevot, V. Phenotyping of nNOS neurons in the postnatal and adult female mouse hypothalamus. J. Comp. Neurol. 2017, 525, 3177–3189. [Google Scholar] [CrossRef]
- Moiseev, K.Y.; Vishnyakova, P.A.; Porseva, V.V.; Masliukov, A.P.; Spirichev, A.A.; Emanuilov, A.I.; Masliukov, P.M. Changes of nNOS expression in the tuberal hypothalamic nuclei during ageing. Nitric Oxide 2020, 100–101, 1–6. [Google Scholar] [CrossRef]
- Dawson, T.M.; Dawson, V.L. Nitric Oxide Signaling in Neurodegeneration and Cell Death. Adv. Pharmacol. 2018, 82, 57–83. [Google Scholar] [CrossRef]
- Stepanichev, M.; Aniol, V.; Lazareva, N.; Gulyaeva, N. Decreased Hippocampal Neurogenesis in Aged Male Wistar Rats Is Not Associated with Memory Acquisition in a Water Maze. Int. J. Mol. Sci. 2023, 24, 13276. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, e2303259. [Google Scholar] [CrossRef]
- Tewari, D.; Sah, A.N.; Bawari, S.; Nabavi, S.F.; Dehpour, A.R.; Shirooie, S.; Braidy, N.; Fiebich, B.L.; Vacca, R.A.; Nabavi, S.M. Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr. Neuropharmacol. 2021, 19, 114–126. [Google Scholar] [CrossRef]
- Carletti, F.; Gambino, G.; Rizzo, V.; Ferraro, G.; Sardo, P. Cannabinoid and nitric oxide signaling interplay in the modulation of hippocampal hyperexcitability: Study on electrophysiological and behavioral models of temporal lobe epilepsy in the rat. Neuroscience 2015, 303, 149–159. [Google Scholar] [CrossRef]
- Belgardt, B.F.; Mauer, J.; Wunderlich, F.T.; Ernst, M.B.; Pal, M.; Spohn, G.; Brönneke, H.S.; Brodesser, S.; Hampel, B.; Schauss, A.C.; et al. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc. Natl. Acad. Sci. USA 2010, 107, 6028–6033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, G.; Zhang, H.; Karin, M.; Bai, H.; Cai, D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008, 135, 61–73. [Google Scholar] [CrossRef]
- Zhang, Y.; Reichel, J.M.; Han, C.; Zuniga-Hertz, J.P.; Cai, D. Astrocytic Process Plasticity and IKKβ/NF-κB in Central Control of Blood Glucose, Blood Pressure, and Body Weight. Cell Metab. 2017, 25, 1091–1102.e4. [Google Scholar] [CrossRef] [PubMed]
- González-García, I.; García-Cáceres, C. Hypothalamic Astrocytes as a Specialized and Responsive Cell Population in Obesity. Int. J. Mol. Sci. 2021, 22, 6176. [Google Scholar] [CrossRef] [PubMed]
- McMurphy, T.; Huang, W.; Liu, X.; Siu, J.J.; Queen, N.J.; Xiao, R.; Cao, L. Hypothalamic gene transfer of BDNF promotes healthy aging in mice. Aging Cell 2019, 18, e12846. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Banno, R.; Shibata, M.; Adachi, K.; Hagimoto, S.; Hagiwara, D.; Ozawa, Y.; Goto, M.; Suga, H.; Sugimura, Y.; et al. GABA type B receptor signaling in proopiomelanocortin neurons protects against obesity, insulin resistance, and hypothalamic inflammation in male mice on a high-fat diet. J. Neurosci. 2013, 33, 17166–71713. [Google Scholar] [CrossRef]
- Yu, B.; Cai, D. Neural Programmatic Role of Leptin, TNFα, Melanocortin, and Glutamate in Blood Pressure Regulation vs Obesity-Related Hypertension in Male C57BL/6 Mice. Endocrinology 2017, 158, 1766–1775. [Google Scholar] [CrossRef] [PubMed]



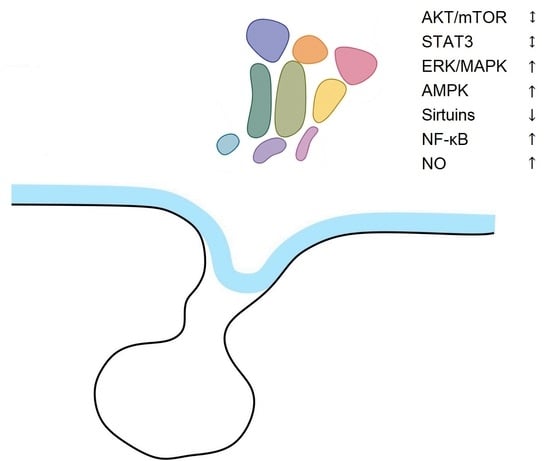
| Signaling Pathway | Aging Tissues | Aging Hypothalamus |
|---|---|---|
| AKT/mTOR | Differential actions in separate organs | No changes |
| STAT3 | No changes | No changes |
| ERK/MAPK | Decreased | Increased |
| AMPK | Decreased | Increased |
| Sirtuins | Decreased | Decreased |
| NF-κB | Increased | Increased |
| NO | Increased | Increased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masliukov, P.M. Changes of Signaling Pathways in Hypothalamic Neurons with Aging. Curr. Issues Mol. Biol. 2023, 45, 8289-8308. https://doi.org/10.3390/cimb45100523
Masliukov PM. Changes of Signaling Pathways in Hypothalamic Neurons with Aging. Current Issues in Molecular Biology. 2023; 45(10):8289-8308. https://doi.org/10.3390/cimb45100523
Chicago/Turabian StyleMasliukov, Petr M. 2023. "Changes of Signaling Pathways in Hypothalamic Neurons with Aging" Current Issues in Molecular Biology 45, no. 10: 8289-8308. https://doi.org/10.3390/cimb45100523
APA StyleMasliukov, P. M. (2023). Changes of Signaling Pathways in Hypothalamic Neurons with Aging. Current Issues in Molecular Biology, 45(10), 8289-8308. https://doi.org/10.3390/cimb45100523