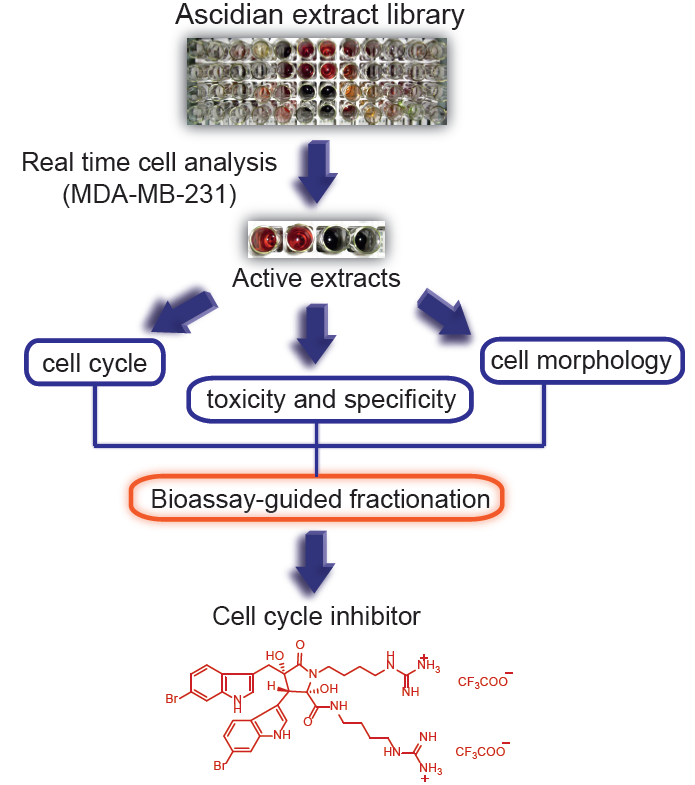
Identification of Eusynstyelamide B as a Potent Cell Cycle Inhibitor Following the Generation and Screening of an Ascidian-Derived Extract Library Using a Real Time Cell Analyzer
Abstract
:1. Introduction
2. Results
2.1. Time-Dependent Cell Response Analysis of Ascidian Extracts by RTCA
| Extract Number | Species |
|---|---|
| 15 | Polysyncraton millepore |
| 17 | Trididemnum cf. cerebriforme |
| 29 | Trididemnum pigmentatum |
| 38 | Polysyncraton echinatum |
| 43 | Leptoclinides kingi |
| 44 | Leptoclinides durus |
| 53 | Trididemnum sibogae |
| 61 | Lissoclinum badium |
| 63 | Polysyncraton pseudorugosum |
| 71 | Leptoclinides dubius |
| 75 | Polysyncraton pseudorugosum |
| 81 | Leptoclinides durus |
| 83 | Leptoclinides dubius |
| 85 | Lissoclinum fungium |
| 92 | Leptoclinides kingi |
| 102 | Didemnum candidum |
| 106 | Didemnum multispirale |
| 114 | Didemnum candidum |
| 117 | Didemnum membranaceum |
| 128 | Didemnum membranaceum |
| 133 | Didemnum guttatum |
2.2. Cell Count after Treatment with Ascidian Extracts
2.3. Analysis of Cell Morphology by Microscopy



2.4. Cell Cycle Studies

2.5. Prioritization, Extraction and Bioassay-Guided Fractionation of Ascidian Extract 114 (Didemnum candidum)
2.6. Determination of IC50 for Eusynstyelamide B (1)
2.7. Validation of 1 by RTCA and Cell Cycle Analysis

2.8. Mode of Cell Death Studies

3. Experimental Section
3.1. General
3.2. Ascidian Material
3.3. Generation of the Ascidian Extract Library
3.4. Cell Culture
3.5. Real Time Cell Analysis of the Ascidian Extract Library
3.6. Cell Counting of the Ascidian Extract Library
3.7. Cell Morphology Analysis by Phase-Contrast Microscopy
3.8. Cell Cycle Studies of the 21 Active Ascidian Extracts
3.9. Large-Scale Extraction of Didemnum candidum (Ascidian Code: 114)
3.10. Bioassay-Guided Fractionation of the Extract from Didemnum candidum (Ascidian Code: 114)
3.11. Determination of the IC50 of Eusynstyelamide B (1)
3.12. Western Blotting Experiments Using Eusynstyelamide B (1)
3.13. Annexin V Assay Using Eusynstyelamide B (1)
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cragg, G.M.L.; Kingston, D.; Newman, D.J. Anticancer Agents from Natural Products; Taylor & Francis/CRC Press: Boca Raton, FL, USA, 2005; p. 577. [Google Scholar]
- Barenbrock, J.S.; Kock, M. Screening enzyme-inhibitory activity in several ascidian species from Orkney Islands using protein tyrosine kinase (PTK) bioassay-guided fractionation. J. Biotechnol. 2005, 117, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Menna, M. Antitumor potential of natural products from Mediterranean ascidians. Phytochem. Rev. 2009, 8, 461–472. [Google Scholar] [CrossRef]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar]
- Houssen, W.E.; Jaspars, M. Isolation of marine natural products. In Natural Products Isolation, 2nd ed.; Sarker, S.D., Latif, Z., Gray, A.I., Eds.; Humana Press: Totowa, NJ, USA, 2006; Volume 20; pp. 353–390. [Google Scholar]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Erwin, P.M.; López-Legentil, S.; Schuhmann, P.W. The pharmaceutical value of marine biodiversity for anti-cancer drug discovery. Ecol. Econ. 2010, 70, 445–451. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. Contributions to the study of marine products. J. Org. Chem. 1951, 16, 981–987. [Google Scholar] [CrossRef]
- Vera, M.D.; Joullié, M.M. Natural products as probes of cell biology: 20 years of didemnin research. Med. Res. Rev. 2002, 22, 102–145. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, K.L.; Holt, T.G.; Fregeau, N.L.; Stroh, J.G.; Keifer, P.A.; Sun, F.; Li, L.H.; Martin, D.G. Ecteinascidins 729, 743, 745, 759A, 759B, and 770: Potent antitumor agents from the Caribbean tunicate Ecteinascidia turbinata. J. Org. Chem. 1990, 55, 4512–4515. [Google Scholar] [CrossRef]
- Wright, A.E.; Forleo, D.A.; Gunawardana, G.P.; Gunasekera, S.P.; Koehn, F.E.; McConnell, O.J. Antitumor tetrahydroisoquinoline alkaloids from the colonial ascidian Ecteinascidia turbinata. J. Org. Chem. 1990, 55, 4508–4512. [Google Scholar] [CrossRef]
- Amador, M.L.; Jimeno, J.; Paz-Ares, L.; Cortes-Funes, H.; Hidalgo, M. Progress in the development and acquisition of anticancer agents from marine sources. Ann. Oncol. 2003, 14, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- SEER Cancer Statistics Review, 1975–2010. Available online: http://seer.cancer.gov/csr/1975_2010/ (accessed on 7 May 2013).
- Breast Cancer in Australia: An Overview; Australian Institute of Health and Welfare: Canberra, Australia, October 2012; p. 187.
- Passant, H.; Borley, A. Adjuvant treatment for breast cancer. Surgery 2013, 31, 37–40. [Google Scholar]
- Hassan, M.S.; Ansari, J.; Spooner, D.; Hussain, S.A. Chemotherapy for breast cancer (Review). Oncol. Rep. 2010, 24, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Subik, K.; Lee, J.F.; Baxter, L.; Strzepek, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.C.; Bonfiglio, T.; Hicks, D.G.; et al. The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer Auckl. 2010, 4, 35–41. [Google Scholar] [PubMed]
- Abassi, Y.A.; Xi, B.; Zhang, W.; Ye, P.; Kirstein, S.L.; Gaylord, M.R.; Feinstein, S.C.; Wang, X.; Xu, X. Kinetic cell-based morphological screening: prediction of mechanism of compound action and off-target effects. Chem. Biol. 2009, 16, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Boleti, A.P.D.; Ventura, C.A.; Justo, G.Z.; Silva, R.A.; de Sousa, A.C.T.; Ferreira, C.V.; Yano, T.; Macedo, M.L.R. Pouterin, a novel potential cytotoxic lectin-like protein with apoptosis-inducing activity in tumorigenic mammalian cells. Toxicon 2008, 51, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Tapiolas, D.M.; Bowden, B.F.; Abou-Mansour, E.; Willis, R.H.; Doyle, J.R.; Muirhead, A.N.; Liptrot, C.; Llewellyn, L.E.; Wolff, C.W.W.; Wright, A.D.; et al. Eusynstyelamides A, B, and C, nNOS Inhibitors, from the Ascidian Eusynstyela latericius. J. Nat. Prod. 2009, 72, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, M.; Tabudravu, J.N.; Jaspars, M.; Strom, M.B.; Hansen, E.; Andersen, J.H.; Kristiansen, P.E.; Haug, T. The antibacterial ent-eusynstyelamide B and eusynstyelamides D, E, and F from the Arctic bryozoan Tegella cf. spitzbergensis. J. Nat. Prod. 2011, 74, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Dictionary of Natural Products (DVD), 18.5v; Taylor & Francis Group/CRC Press: London, UK, 2013.
- Decant, W. Molecular, clinical and environmental toxicology. In Molecular Toxicology; Luch, A., Ed.; Birkhauser Verlag AG: Basel, Switzerland, 2009; Volume 1; p. 470. [Google Scholar]
- Dias, N.; Vezin, H.; Lansiaux, A.; Bailly, C. Topoisomerase inhibitors of marine origin and their potential use as anticancer asgents. In DNA Binders and Related Subjects; Waring, M., Chaires, J., Eds.; Springer: Berlin Heidelberg, Germany, 2005; Volume 253; pp. 89–108. [Google Scholar]
- Menna, M.; Fattorusso, E.; Imperatore, C. Alkaloids from marine ascidians. Molecules 2011, 16, 8694–8732. [Google Scholar] [CrossRef] [Green Version]
- Swersey, J.C.; Ireland, C.M.; Cornell, L.M.; Peterson, R.W. Eusynstyelamide, a highly modified dimer peptide from the ascidian Eusynstyela misakiensis. J. Nat. Prod. 1994, 57, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Barykina, O.V.; Snider, B.B. Synthesis of (+/−)-eusynstyelamide A. Org. Lett. 2010, 12, 2664–2667. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.W.; Donia, M.S. Life in cellulose houses: Symbiotic bacterial biosynthesis of ascidian drugs and drug leads. Curr. Opin. Biotechnol. 2010, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Fricke, W.F.; Partensky, F.; Cox, J.; Elshahawi, S.I.; White, J.R.; Phillippy, A.M.; Schatz, M.C.; Piel, J.; Haygood, M.G.; et al. Complex microbiome underlying secondary and primary metabolism in the tunicate-Prochloron symbiosis. Proc. Natl. Acad. Sci. USA 2011, 108, E1423–E1432. [Google Scholar] [CrossRef] [PubMed]
- Matthews, N.E.; Adams, M.A.; Maxwell, L.R.; Gofton, T.E.; Graham, C.H. Nitric oxide-mediated regulation of chemosensitivity in cancer cells. J. Natl. Cancer Inst. 2001, 93, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liberio, M.S.; Sadowski, M.C.; Nelson, C.C.; Davis, R.A. Identification of Eusynstyelamide B as a Potent Cell Cycle Inhibitor Following the Generation and Screening of an Ascidian-Derived Extract Library Using a Real Time Cell Analyzer. Mar. Drugs 2014, 12, 5222-5239. https://doi.org/10.3390/md12105222
Liberio MS, Sadowski MC, Nelson CC, Davis RA. Identification of Eusynstyelamide B as a Potent Cell Cycle Inhibitor Following the Generation and Screening of an Ascidian-Derived Extract Library Using a Real Time Cell Analyzer. Marine Drugs. 2014; 12(10):5222-5239. https://doi.org/10.3390/md12105222
Chicago/Turabian StyleLiberio, Michelle S., Martin C. Sadowski, Colleen C. Nelson, and Rohan A. Davis. 2014. "Identification of Eusynstyelamide B as a Potent Cell Cycle Inhibitor Following the Generation and Screening of an Ascidian-Derived Extract Library Using a Real Time Cell Analyzer" Marine Drugs 12, no. 10: 5222-5239. https://doi.org/10.3390/md12105222
APA StyleLiberio, M. S., Sadowski, M. C., Nelson, C. C., & Davis, R. A. (2014). Identification of Eusynstyelamide B as a Potent Cell Cycle Inhibitor Following the Generation and Screening of an Ascidian-Derived Extract Library Using a Real Time Cell Analyzer. Marine Drugs, 12(10), 5222-5239. https://doi.org/10.3390/md12105222