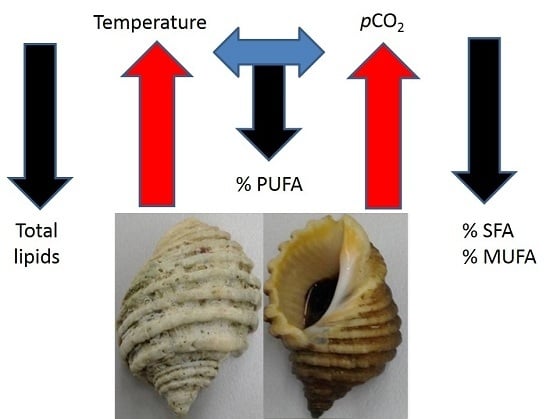
Ocean Warming and CO2-Induced Acidification Impact the Lipid Content of a Marine Predatory Gastropod
Abstract
:1. Introduction
2. Results and Discussion
2.1. Impacts of Ocean Climate Change on D. orbita Total Lipid Content

| Temperature | Acidification | Temperature × Acidification | ||||
|---|---|---|---|---|---|---|
| Pseudo F | p Value | Pseudo F | p Value | Pseudo F | p Value | |
| UNIVARIATE | ||||||
| Total lipid yield | 19.1230 | 0.0002 | 1.9863 | 0.1796 | 1.6396 | 0.2098 |
| SFA | 3.6189 | 0.0733 | 4.2989 | 0.0478 | 3.1075 | 0.0905 |
| MUFA | 0.4115 | 0.5451 | 5.7182 | 0.0196 | 0.0063 | 0.9373 |
| PUFA | 10.981 | 0.0029 | 0.0281 | 0.8705 | 6.7298 | 0.0164 |
| n-3 | 4.7150 | 0.0476 | 0.0002 | 0.9881 | 1.8572 | 0.1848 |
| n-6 | 5.1551 | 0.0340 | 1.2660 | 0.2742 | 0.0452 | 0.8261 |
| n-3:n-6 ratio | 0.33697 | 0.5697 | 0.20287 | 0.6637 | 1.0828 | 0.3088 |
| MULTIVARIATE | ||||||
| Overall fatty acid composition | 7.7094 | 0.0001 | 2.8452 | 0.0229 | 1.7186 | 0.1321 |
2.2. Impacts of Ocean Climate Change on the Major Classes of Fatty Acids

| Fatty Acid | Trivial Name | Retention Time (min) | 23 °C, Current pCO2 | 23 °C, Future pCO2 | 25 °C, Current pCO2 | 25 °C, Future pCO2 |
|---|---|---|---|---|---|---|
| Saturated | ||||||
| C14:0 | Myristic | 16.8 | 1.46 ± 0.13 | 1.56 ± 0.05 | 1.74 ± 0.08 | 1.28 ± 0.12 |
| C15:0 | Pentadecanoic | 18.4 | 1.24 ± 0.18 | 1.30 ± 0.07 | 1.38 ± 0.11 | 1.11 ± 0.12 |
| C16:0 | Palmitic | 19.8 | 9.26 ± 0.49 | 8.86 ± 0.17 | 9.53 ± 0.39 | 8.66 ± 0.23 |
| C17:0 | Margaric | 21.3 | 1.98 ± 0.12 | 1.71 ± 0.10 | 1.31 ± 0.12 | 1.22 ± 0.13 |
| C18:0 | Stearic | 22.6 | 8.26 ± 0.49 | 8.17 ± 0.15 | 7.90 ± 0.24 | 8.30 ± 0.12 |
| C24:0 | Lignoceric | 29.7 | 4.54 ± 0.16 | 4.99 ± 0.36 | 4.81 ± 0.16 | 4.18 ± 0.13 |
| Monounsaturated | ||||||
| C16:1 | Palmitoleic | 20.6 | 1.87 ± 0.16 | 0.84 ± 0.32 | 0.89 ± 0.25 | 0.86 ± 0.23 |
| C18:1 (n-9) | Oleic | 23.1 | 4.54 ± 0.49 | 4.96 ± 0.07 | 4.89 ± 0.23 | 4.42 ± 0.06 |
| C20:1 (n-9) | 11-Eicosenoic | 25.7 | 3.50 ± 0.11 | 3.40 ± 0.19 | 3.81 ± 0.16 | 3.81 ± 0.19 |
| C22:1 (n-9) | Erucic | 30.2 | 0.24 ± 0.04 | 0.20 ± 0.01 | 0.34 ± 0.11 | 0.15 ± 0.07 |
| Polyunsaturated | ||||||
| C18:2 (n-6) | Linoleic acid (LA) | 24.1 | 1.54 ± 0.09 | 1.51 ± 0.07 | 1.66 ± 0.12 | 1.69 ± 0.08 |
| C18:3 (n-3) | α-Linolenic (ALA) | 25.1 | 0.56 ± 0.04 | 0.60 ± 0.03 | 0.69 ± 0.06 | 0.77 ± 0.04 |
| C20:2 | Eicosadienoic | 26.5 | 2.50 ± 0.13 | 2.89 ± 0.16 | 2.60 ± 0.1 | 2.45 ± 0.13 |
| C20:3 (n-3) | Eicosatrienoic | 27.1 | 0.03 ± 0.03 | 0.03 ± 0.03 | 0 | 0 |
| C20:4 (n-6) | Arachidonic (ARA) | 27.3 | 11.49 ± 0.22 | 11.84 ± 0.26 | 10.88 ± 0.16 | 11.07 ± 0.14 |
| C20:5 (n-3) | Eicosapentaenoic (EPA) | 28.3 | 2.61 ± 0.30 | 2.76 ± 0.33 | 2.00 ± 0.42 | 2.05 ± 0.44 |
| C22:2 | Docosadienoic | 28.5 | 11.15 ± 0.57 | 10.15 ± 0.39 | 11.81 ± 0.51 | 11.40 ± 0.42 |
| C22:5 (n-3) | Docosapentaenoic (DPA) | 30.7 | 17.72 ± 0.49 | 16.87 ± 0.52 | 16.13 ± 0.40 | 16.69 ± 0.39 |
| C22:6 (n-3) | Docosahexaenoic (DHA) | 30.9 | 4.02 ± 0.27 | 3.70 ± 0.13 | 3.55 ± 0.26 | 3.85 ± 0.35 |
| Others | ||||||
| 2-octylcyclo-propanedecanoic | 26.8 | 0.63 ± 0.02 | 0.64 ± 0.04 | 0.68 ± 0.03 | 0.73 ± 0.02 | |
| Unknown fatty acid derivative | 29.2 | 0.53 ± 0.04 | 0.48 ± 0.06 | 0.56 ± 0.05 | 0.50 ± 0.05 | |
| Dimethyl acetal aldehydes | ||||||
| Hexadecan-1-al | 18.8 | 1.04 ± 0.08 | 0.94 ± 0.04 | 0.87 ± 0.70 | 0.82 ± 0.02 | |
| Heptadecan-1-al | 20.2 | 0.21 ± 0.05 | 1.03 ± 0.26 | 0.86 ± 0.17 | 1.78 ± 0.06 | |
| Octadecan-1-al | 21.7 | 8.91 ± 0.83 | 10.27 ± 0.20 | 10.80 ± 0.29 | 11.92 ± 0.20 | |
| Nonadecan-1-al | 24.7 | 0.17 ± 0.06 | 0.30 ± 0.03 | 0.30 ± 0.07 | 0.29 ± 0.06 | |
2.3. Impacts of Ocean Climate Change on Fatty Acid Composition

3. Experimental Section
3.1. Study Site and Experimental Design
3.2. Extraction and Preparation of Fatty Acid Methyl Esters
3.3. FAMEs and GC Analysis
3.4. Statistical Analyses
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- IPCC. Climate Change 2007. The Physical Science Basis. Contribution of Working Group 1 to the Fourth Assessment, Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Caldeira, K.; Wickett, M.E. Oceanography: Anthropogenic carbon and ocean pH. Nature 2003, 425, 365–365. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: Vulnerabilities and potential for persistence in a changing ocean. Oceanogr. Mar. Biol. 2011, 49, 1–42. [Google Scholar]
- Harley, C.D.G.; Denny, M.W.; Mach, K.J.; Miller, L.P. Thermal stress and morphological adaptations in limpets. Funct. Ecol. 2009, 23, 293–301. [Google Scholar] [CrossRef]
- Nguyen, K.D.T.; Morley, S.A.; Lai, C.H.; Clark, M.S.; Tan, K.S.; Bates, A.E.; Peck, L.S. Upper temperature limits of tropical marine ectotherms: Global warming implications. PLoS ONE 2012, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, L.M.; Ross, P.M.; Pörtner, H.O.; Scanes, E.; Wright, J.M. Predicting the response of molluscs to the impact of ocean acidification. Biology 2013, 2, 651–692. [Google Scholar] [CrossRef]
- Fabry, V.J.; Seibel, B.A.; Feely, R.A.; Orr, J.C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 2008, 65, 414–432. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [PubMed]
- Wittmann, A.C.; Pörtner, H. Sensitivities of extant animal taxa to ocean acidification. Nat. Clim. Chang. 2013, 3, 995–1001. [Google Scholar]
- Hooper, C.; Day, R.; Slocombe, R.; Benkendorff, K.; Handlinger, J.; Goulias, J. Effects of severe heat stress on immune function, biochemistry and histopathology in farmed Australian abalone (hybrid Haliotis laevigata × Haliotis rubra). Aquaculture 2014, 432, 26–37. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Duarte, C.M.; Gattuso, J.P. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Przeslawski, R. Multistressor impacts of warming and acidification of the ocean on marine invertebrates’ life histories. Integr. Comp. Biol. 2013, 53, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Leiva, G.E.; Castilla, J.C. A review of the world marine gastropod fishery: Evolution of catches, management and the Chilean experience. Rev. Fish Biol. Fish. 2002, 11, 283–300. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2012. [Google Scholar]
- Ponder, W.; Hutchings, P.; Chapman, R. Overview of the Conservation of Australian Marine Invertebrates; A Report for Environment Australia; Australian Museum: Sydney, Australia, 2002. [Google Scholar]
- Szabó, K.; Amesbury, J.R. Molluscs in a world of islands: The use of shellfish as a food resource in the tropical island Asia-Pacific region. Quat. Int. 2011, 239, 8–18. [Google Scholar] [CrossRef]
- Koutsoubas, D.; Galinou-Mitsoudi, S.; Katsanevakis, S.; Leontarakis, P.; Metaxatos, A.; Zenetos, A. Bivalve and gastropod molluscs of commercial interest for human consumption in the Hellenic seas. In State of the Hellenic Fisheries; Papaconstantinou, C., Zenetos, A., Tserpes, G., Vassilopoulou, V., Eds.; HCMR Publications: Athens, Greece, 2007; pp. 70–84. [Google Scholar]
- Flores-Garza, R.; García-Ibáñez, S.; Flores-Rodríguez, P.; Torreblanca-Ramírez, C.; Galeana-Rebolledo, L.; Valdés-González, A.; Suástegui-Zárate, A.; Violante-González, J. Commercially important marine mollusks for human consumption in Acapulco, México. Nat. Res. 2012, 3, 11–17. [Google Scholar]
- Saito, H.; Aono, H. Characteristics of lipid and fatty acid of marine gastropod Turbo cornutus: High levels of arachidonic and n-3 docosapentaenoic acid. Food Chem. 2014, 145, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.D.; Virtue, P.; Mooney, B.D.; Elliott, N.G.; Yearsley, G.K. Seafood the Good Food. The Oil (Fat) Content and Composition of Australian Commercial Fishes, Shellfishes and Crustaceans; CSIRO Division of Marine Research; Fisheries Research and Development Corporation: Deakin, Australia, 1998. [Google Scholar]
- Ruxton, C.H.S.; Reed, S.C.; Simpson, M.J.A.; Millington, K.J. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J. Hum. Nutr. Diet. 2007, 20, 275–285. [Google Scholar] [CrossRef]
- Meyer, B.J.; Mann, N.J.; Lewis, J.L.; Milligan, G.C.; Sinclair, A.J.; Howe, P.R.C. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 2003, 38, 391–398. [Google Scholar] [CrossRef]
- Covington, M.B. Omega-3 fatty acids. Am. Fam. Physician 2004, 70, 133–140. [Google Scholar] [PubMed]
- Kimura, Y.; Kono, S.; Toyomura, K.; Nagano, N.; Mizoue, T.; Moore, M.A.; Mibu, R.; Tanaka, M.; Kakeji, Y.; Maehara, Y.; et al. Meat, fish and fat intake in relation to subsite-specific risk of colorectal cancer: The Fukuoka colorectal cancer study. Cancer Sci. 2007, 98, 590–597. [Google Scholar] [PubMed]
- De Wilde, M.; Hogyes, E.; Kiliaan, A.J.; Farkas, T.; Luiten, P.G.M.; Farkas, E. Dietary fatty acids alter blood pressure, behavior and brain membrane composition of hypertensive rats. Brain Res. 2003, 988, 9–19. [Google Scholar] [CrossRef]
- Kendall-Tackett, K. Long-chain omega-3 fatty acids and women’s mental health in the perinatal period and beyond. J. Midwifery Womens Health 2010, 55, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzia, V.; D’Intronoa, A.; Colaciccoa, A.M.; Capursob, C.; del Parigic, A.; Capursoa, S.; Gadaletaa, A.; Capursoa, A.; Panzaa, F. Dietary fatty acids intake: Possible role in cognitive decline and dementia. Exp. Gerontol. 2005, 40, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Leelarthaepin, B.; Majchrzak-Hong, S.F.; Faurot, K.R.; Suchindran, C.M.; Ringel, A.; Davis, J.M.; Hibbeln, J.R. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney diet heart study and updated meta-analysis. BMJ 2013, 346, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Harvey, N.C.; Robinson, S.M.; Ntani, G.; Davies, J.H.; Inskip, H.M.; Godfrey, K.M.; Dennison, E.M.; Calder, P.C.; Cooper, C.; et al. Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J. Clin. Endocrinol. Metab. 2013, 98, 299–307. [Google Scholar] [PubMed]
- Noyola, J.; Mascaró, M.; Caamal-Monsreal, C.; Noreña-Barroso, E.; Díaz, F.; Re, D.; Sánchez, A.; Rosas, C. Effect of temperature on energetic balance and fatty acid composition of early juveniles of Octopus maya. J. Exp. Mar. Biol. Ecol. 2013, 445, 156–165. [Google Scholar] [CrossRef]
- Rossoll, D.; Bermudez, R.; Hauss, H.; Schulz, K.G.; Riebesell, U.; Sommer, U.; Winder, M. Ocean acidification-induced food quality deterioration constrains trophic transfer. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagenen, J.V.; Miller, T.W.; Hobbs, S.; Hook, P.; Crowe, B.; Huesemann, M. Effects of light and temperature on fatty acid production in Nannochloropsis salina. Energies 2012, 5, 731–740. [Google Scholar] [CrossRef]
- Farkas, T.; Herodek, S. The effect of environmental temperature on the fatty acid composition of crustacean plankton. J. Lipid Res. 1964, 5, 369–373. [Google Scholar] [PubMed]
- Thompson, P.A.; Guo, M.; Harrison, P.J.; Whyte, J.N.C. Effects of variation in temperature. II. On the fatty acid composition of eight species of marine phytoplankton. J. Phycol. 1992, 28, 488–497. [Google Scholar]
- Nanton, D.A.; Castell, J.D. The effects of temperature and dietary fatty acids on the fatty acid composition of harpacticoid copepods, for use as a live food for marine fish larvae. Aquaculture 1999, 175, 167–181. [Google Scholar] [CrossRef]
- Renaud, S.M.; Zhou, H.C.; Parry, D.L.; Thinh, L.V.; Woo, K.C. Effect of temperature on the growth, total lipid content and fatty acid composition of recently isolated tropical microalgae Isochrysis sp., Nitzschia closterium, Nitzschia paleacea, and commercial species Isochrysis sp. (clone T.ISO). J. Appl. Phycol. 1995, 7, 595–602. [Google Scholar]
- Sushchik, N.N.; Kalacheva, G.S.; Zhila, N.O.; Gladyshev, M.I.; Volova, T.G. A temperature dependence of the intra- and extracellular fatty-acid composition of green algae and cyanobacterium. Russ. J. Plant Physiol. 2003, 50, 374–380. [Google Scholar] [CrossRef]
- Allen, E.E.; Facciotti, D.; Bartlett, D.H. Monounsaturated but not polyunsaturated fatty acids are required for growth of the deep-sea bacterium Photobacterium profundum ss9 at high pressure and low temperature. Appl. Environ. Microbiol. 1999, 65, 1710–1720. [Google Scholar] [PubMed]
- Przytulska, A.; Bartosiewicz, M.; Rautio, M.; Dufresne, F.; Vincent, W.F. Climate effects on high latitude Daphnia via food quality and thresholds. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.W. Temperature and pressure effects on the fatty acids of some marine ectotherms. Comp. Biochem. Physiol. 1962, 6, 75–89. [Google Scholar] [CrossRef]
- Fishery and Aquaculture Statistics. FishStatJ—Software for Fishery Statistical Time Series. Available online: http://www.Fao.Org/fishery/statistics/software/fishstatj/en (accessed on 4 April 2015).
- Woodcock, S.H.; Benkendorff, K. The impact of diet on the growth and proximate composition of juvenile whelks, Dicathais orbita (Gastropoda:Mollusca). Aquaculture 2008, 276, 162–170. [Google Scholar] [CrossRef]
- Naegel, L.C.A.; Cooksey, C. Tyrian purple from marine muricids, especially from Plicopurpura pansa (Gould, 1853). J. Shellfish Res. 2002, 21, 193–200. [Google Scholar]
- Benkendorff, K. Aquaculture and the production of pharmaceuticals and nutraceuticals. In New Technologies in Aquaculture; Burnell, G., Allan, G., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 866–891. [Google Scholar]
- Benkendorff, K. Natural products in the Australian marine invertebrate Dicathais orbita. Mar. Drugs 2013, 11, 1370–1398. [Google Scholar] [CrossRef] [PubMed]
- Bech, M. A survey of imposex in muricids from 1996 to 2000 and identification of optimal indicators of tributyltin contamination along the east coast of Phuket Island, Thailand. Mar. Pollut. Bull. 2002, 44, 887–896. [Google Scholar] [CrossRef]
- Gibson, C.P.; Wilson, S.P. Imposex still evident in eastern Australia 10 years after tributyltin restrictions. Mar. Environ. Res. 2003, 55, 101–112. [Google Scholar] [CrossRef]
- Bech, M.; Strand, J.; Jacobsen, J.A. Development of imposex and accumulation of butyltin in the tropical muricid Thais distinguenda transplanted to a TBT contaminated site. Environ. Pollut. 2002, 119, 253–260. [Google Scholar] [CrossRef]
- Talmage, S.S.; Gobler, C.J. Effects of elevated temperature and carbon dioxide on the growth and survival of larvae and juveniles of three species of Northwest Atlantic bivalves. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Rais, A.; Miller, N.; Stillman, J.H. No evidence for homeoviscous adaptation in intertidal snails: Analysis of membrane fluidity during thermal acclimation, thermal acclimatization, and across thermal microhabitats. Mar. Biol. 2010, 157, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Hazel, J.R. Thermal adaptation in biological membranes: Is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 1995, 57, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, N.V. Lipid classes and fatty acid composition of the tropical nudibranch mollusks Chromodoris sp. and Phyllidia coelestis. Lipids 2007, 42, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Todd, C.D.; Hughes, S.L.; Marshall, C.T.; MaClean, J.C.; Lonergan, M.E.; Biuw, E.M. Detrimental effects of recent ocean surface warming on growth condition of Atlantic salmon. Glob. Chang. Biol. 2008, 14, 1–13. [Google Scholar]
- Hobday, A.J.; Pecl, G.T. Identification of global marine hotspots: Sentinels for change and vanguards for adaptation action. Rev. Fish Biol Fish. 2014, 24, 415–425. [Google Scholar] [CrossRef]
- Somero, G.N. The physiology of climate change: How potentials for acclimatization and genetic adaptation will determine “winners” and “losers”. J. Exp. Biol. 2009, 213, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.A.; Dworjanyn, S.A.; Poore, A.G.B.; Byrne, M. Adaptive capacity of the habitat modifying sea urchin Centrostephanus rodgersii to ocean warming and ocean acidification: Performance of early embryos. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Zarai, Z.; Frikha, F.; Balti, R.; Miled, N.; Gargouri, Y.; Mejdoub, H. Nutrient composition of the marine snail (Hexaplex trunculus) from the Tunisian Mediterranean coasts. J. Sci. Food Agric. 2011, 91, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Yannakopoulos, A.; Tserveni-Gousi, A.; Christaki, E. Enhanced egg production in practice: The case of bio-omega-3 egg. Int. J. Poult. Sci. 2005, 4, 531–535. [Google Scholar]
- Milinsk, M.C.; Padre, R.G.; Hayashi, C.; Souza, N.E.; Matsushita, M. Influence of diets enriched with different vegetable oils on the fatty acid profiles of Helix aspersa maxima. Food Chem. 2003, 82, 553–558. [Google Scholar] [CrossRef]
- Simopoulus, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560S–569S. [Google Scholar]
- Anacleto, P.; Maulvault, A.L.; Bandarra, N.M.; Repolho, T.; Nunes, M.L.; Rosa, R.; Marques, A. Effect of warming on protein, glycogen and fatty acid content of native and invasive clams. Food Res. Int. 2014, 64, 439–445. [Google Scholar] [CrossRef]
- Schiffman, E.T.; Coffey, W.D.; Hua, W.; Nunn, B.L.; Dickinson, G.H.; Roberts, S.B. Shotgun proteomics reveals physiological response to ocean acidification in Crassostrea gigas. BMC Genom. 2014, 15. [Google Scholar] [CrossRef]
- Sato, N.; Tsuzuki, M.; Kawaguchi, A. Glycerolipid synthesis in Chlorella kessleri 11 h II. Effect of the CO2 concentration during growth. Biochim. Biophys. Acta 2003, 1633, 35–42. [Google Scholar] [PubMed]
- Pörtner, H. Ecosystem effects of ocean acidification in times of ocean warming: A physiologist’s view. Mar. Ecol. Prog. Ser. 2008, 373, 203–217. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Rob, T.; Ogi, T.; Maarisit, W.; Taira, J.; Ueda, K. Isolation of C11 compounds and a cyclopropane fatty acid from an Okinawan ascidian, Diplosoma sp. Molecules 2011, 16, 9972–9982. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, N.M.; Montano, N.; Vicente, J.; Rodriguez, A.D. Novel cyclopropane fatty acids from the phospholipids of the Caribbean sponge Pseudospongosorites suberitoides. Lipids 2007, 42, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Misra, K.K.; Shkrob, I.; Rakshit, S.; Dembitsky, V.M. Variability in fatty acids and fatty aldehydes in different ograns of two prosobranch gastropod mollusks. Biochem. Syst. Ecol. 2002, 30, 749–761. [Google Scholar] [CrossRef]
- Brown, J.L.; Ross, T.; McMeekin, T.A.; Nichols, P.D. Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance. Int. J. Food Microbiol. 1997, 37, 163–173. [Google Scholar] [CrossRef]
- Denich, T.J.; Beaudette, L.; Lee, H.; Trevors, J.T. Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J. Microbiol. Methods 2003, 52, 149–182. [Google Scholar] [CrossRef]
- Christie, W. Mass Spectra of Some Miscellaneous Lipophylic Components. Available online: http://lipidlibrary.aocs.org/Analysis/content.cfm?ItemNumber=39508 (accessed on 18 September 2015).
- Nagan, N.; Zoeller, R.A. Plasmalogens: Biosynthesis and functions. Prog. Lipid Res. 2001, 40, 199–229. [Google Scholar] [CrossRef]
- Munro, D.; Blier, P.U. Age, diet, and season do not affect longevity-related differences in peroxidation index between Spisula solidissima and Arctica islandica. J. Gerontol. Biol. Sci. 2015, 70, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Navy Metoc. Coastal Sea Surface Temperatures. Available online: http://www.metoc.gov.au/products/data/aussst.php (accessed on 25 April 2015).
- Poore, A.G.; Graba-Landry, A.; Favret, M.; Sheppard Brennand, H.; Byrne, M.; Dworjanyn, S.A. Direct and indirect effects of ocean acidification and warming on a marine plant-herbivore interaction. Oecologia 2013, 173, 1113–1124. [Google Scholar] [CrossRef]
- IPCC. IPCC Summary for Policy Makers. In Climate Change 2013: The Physical Science Basis; Contribution of Working Group 1 to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Pierrot, D.; Lewis, E.; Wallace, D.W.R. MS Excel Program Developed for CO2 System Calculations, Ornl/Cdiac-105; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory U.S. Department of Energy: Oak Ridge, TN, USA, 2006. [Google Scholar]
- Mehrbach, C.; Culberson, C.H.; Hawley, J.E.; Pytkowicz, R.M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 1973, 18, 897–907. [Google Scholar] [CrossRef]
- Dickson, A.G.; Millero, F.J. A comparison of the equilibrium-constants for the dissociation of carbonic-acid in seawater media. Deep Sea Res. Part A Oceanogr. Res. Pap. 1987, 34, 1733–1743. [Google Scholar] [CrossRef]
- Kanthilatha, N.; Boyd, W.; Dowell, A.; Mann, A.; Chang, N.; Wolhlmuth, H.; Parr, J. Identification of preserved fatty acids in archeological floor sediments from prehistoric sites at Ban Non Wat and Nong Hua Raet in Northeast Thailand using gas chromatography. J. Archaeol. Sci. 2014, 46, 353–362. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valles-Regino, R.; Tate, R.; Kelaher, B.; Savins, D.; Dowell, A.; Benkendorff, K. Ocean Warming and CO2-Induced Acidification Impact the Lipid Content of a Marine Predatory Gastropod. Mar. Drugs 2015, 13, 6019-6037. https://doi.org/10.3390/md13106019
Valles-Regino R, Tate R, Kelaher B, Savins D, Dowell A, Benkendorff K. Ocean Warming and CO2-Induced Acidification Impact the Lipid Content of a Marine Predatory Gastropod. Marine Drugs. 2015; 13(10):6019-6037. https://doi.org/10.3390/md13106019
Chicago/Turabian StyleValles-Regino, Roselyn, Rick Tate, Brendan Kelaher, Dale Savins, Ashley Dowell, and Kirsten Benkendorff. 2015. "Ocean Warming and CO2-Induced Acidification Impact the Lipid Content of a Marine Predatory Gastropod" Marine Drugs 13, no. 10: 6019-6037. https://doi.org/10.3390/md13106019
APA StyleValles-Regino, R., Tate, R., Kelaher, B., Savins, D., Dowell, A., & Benkendorff, K. (2015). Ocean Warming and CO2-Induced Acidification Impact the Lipid Content of a Marine Predatory Gastropod. Marine Drugs, 13(10), 6019-6037. https://doi.org/10.3390/md13106019