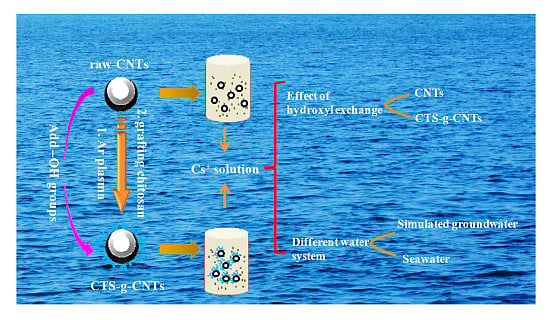
Design of Chitosan-Grafted Carbon Nanotubes: Evaluation of How the –OH Functional Group Affects Cs+ Adsorption
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Characterization




| C=C (%) | C–C (%) | C–O (%) | |
|---|---|---|---|
| raw-CNTs | 74.2 | 15.5 | 10.3 |
| CNTs-treated | 67.5 | 23.7 | 8.8 |
| CTS-g-CNTs | 60 | 17.8 | 22.2 |

2.2. Adsorption Experiment


| Materials | Competitive Cations | Kd (mL/g) | References |
|---|---|---|---|
| CTS-g-CNTs | 0.1 M Li+ | 152.8 | This work |
| 0.1 M Na+ | 118.6 | ||
| 0.1 M K+ | 94.7 | ||
| CA | 3.5 mM Na+ | 69.8 | [ 42] |
| 2.1 mM K+ | 66.5 | ||
| IA | 3.5 mM Na+ | 43.2 | [ 42] |
| 2.1 mM K+ | 26.6 | ||
| PB-coated MNP | 0.1 M Na+ | 56.4 | [ 43] |
| 0.1 M Mg2+ | 112.5 | ||
| 0.1 M K+ | 14.3 |

| System | Sorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| Qmax (mmol/g) | KL (L/mmol) | R2 | n | KF (mmol/g) | R2 | ||
| Simulated groundwater | raw-CNTs | 0.224 | 7.62 | 0.976 | 2.55 | 0.209 | 0.951 |
| CTS-g-CNTs | 0.340 | 3.67 | 0.988 | 1.97 | 0.279 | 0.944 | |
| Seawater | CTS-g-CNTs | 0.272 | 4.79 | 0.987 | 2.30 | 0.242 | 0.986 |
3. Experimental Section
3.1. Materials
3.2. Synthesis of CTS-g-CNTs
3.3. Cesium Adsorption Experiment


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Datta, S.J.; Moon, W.K.; Choi, D.Y.; Hwang, I.C.; Yoon, K.B. A novel vanadosilicate with hexadeca-coordinated Cs+ ions as a highly effective Cs+ remover. Angew. Chem. Int. Ed. 2014, 53, 7203–7208. [Google Scholar] [CrossRef]
- Yang, S.; Han, C.; Wang, X.; Nagatsu, M. Characteristics of cesium ion sorption from aqueous solution on bentonite- and carbon nanotube-based composites. J. Hazard. Mater. 2014, 274, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Fujimoto, K.; Dai, W.; Hunger, M. Molecular mechanism of heavily adhesive Cs: Why radioactive Cs is not decontaminated from soil. J. Phys. Chem. C 2013, 117, 14075–14080. [Google Scholar] [CrossRef]
- Tanaka, K.; Sakaguchi, A.; Kanai, Y.; Tsuruta, H.; Shinohara, A.; Takahashi, Y. Heterogeneous distribution of radiocesium in aerosols, soil and particulate matters emitted by the Fukushima Daiichi Nuclear Power Plant accident: Retention of micro-scale heterogeneity during the migration of radiocesium from the air into ground and river systems. J. Radioanal. Nucl. Chem. 2013, 295, 1927–1937. [Google Scholar] [CrossRef]
- Mizuno, T.; Kubo, H. Overview of active cesium contamination of freshwater fish in Fukushima and eastern Japan. Sci. Rep. 2013, 3, 1–4. [Google Scholar] [CrossRef]
- Yang, D.; Sarina, S.; Zhu, H.; Liu, H.; Zheng, Z.; Xie, M.; Smith, S.V.; Komarneni, S. Capture of radioactive cesium and iodide ions from water by using titanate nanofibers and nanotubes. Angew. Chem. Int. Ed. 2011, 50, 10594–10598. [Google Scholar] [CrossRef]
- Celestian, A.J.; Kubicki, J.D.; Hanson, J.; Clearfield, A.; Parise, J.B. The mechanism responsible for extraordinary Cs ion selectivity in crystalline silicotitanate. J. Am. Chem. Soc. 2008, 130, 11689–11694. [Google Scholar] [CrossRef] [PubMed]
- Torad, N.L.; Hu, M.; Imura, M.; Naito, M.; Yamauchi, Y. Large Cs adsorption capability of nanostructured Prussian Blue particles with high accessible surface areas. J. Mater. Chem. 2012, 22, 18261–18267. [Google Scholar] [CrossRef]
- Dwivedi, C.; Kumar, A.; Ajish, J.K.; Singh, K.K.; Kumar, M.; Wattal, P.K.; Bajaj, P.N. Resorcinol-formaldehyde coated XAD resin beads for removal of cesium ions from radioactive waste: Synthesis, sorption and kinetic studies. RSC Adv. 2012, 2, 5557–5564. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Carbon nanotubes as superior sorbent for dioxin removal. J. Am. Chem. Soc. 2001, 123, 2058–2059. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, T.; Mezzenga, R.; Geckeler, K.E. Carbon nanotubes in the liquid phase: Addressing the issue of dispersion. Small 2012, 8, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.M.; Chen, C.L.; Nagatsu, M.; Wang, X.K. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Belloni, F.; Kütahyali, C.; Rondinella, V.V.; Carbol, P.; Wiss, T.; Mangione, A. Can carbon nanotubes play a role in the field of nuclear waste management? Environ. Sci. Technol. 2009, 43, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Yavari, R.; Huang, Y.D.; Ahmadi, S.J. Adsorption of cesium(I) from aqueous solution using oxidized multiwall carbon nanotubes. J. Radioanal. Nucl. Chem. 2011, 287, 393–401. [Google Scholar] [CrossRef]
- Kaper, H.; Nicolle, J.; Cambedouzou, J.; Grandjean, A. Multi-method analysis of functionalized single-walled carbon nanotubes for cesium liquid–solid extraction. Mater. Chem. Phys. 2014, 147, 147–154. [Google Scholar] [CrossRef]
- Yu, X.Y.; Luo, T.; Zhang, Y.X.; Jia, Y.; Zhu, B.J.; Fu, X.C.; Liu, J.H.; Huang, X.J. Adsorption of lead(II) on O2-plasma-oxidized multiwalled carbon nanotubes: Thermodynamics, kinetics, and desorption. ACS Appl. Mater. Interfaces 2011, 3, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.B.; Shao, D.D.; Wang, X.K.; Nagatsu, M. Localized in situ polymerization on carbon nanotube surfaces for stabilized carbon nanotube dispersions and application for cobalt(II) removal. RSC Adv. 2014, 4, 4856–4863. [Google Scholar] [CrossRef]
- Chen, C.L.; Ogino, A.; Wang, X.K.; Nagatsu, M. Plasma treatment of multiwall carbon nanotubes for dispersion improvement in water. Appl. Phys. Lett. 2010, 9. [Google Scholar] [CrossRef]
- Shao, D.D.; Hu, J.; Wang, X.K. Plasma induced grafting multiwalled carbon nanotube with chitosan and its application for removal of UO22+, Cu2+, and Pb2+ from aqueous solutions. Plasma Process. Polym. 2010, 7, 977–985. [Google Scholar] [CrossRef]
- Swayampakula, K.; Boddu, V.M.; Nadavala, S.K.; Abburi, K. Competitive adsorption of Cu(II), Co(II) and Ni(II) from their binary and tertiary aqueous solutions using chitosan-coated perlite beads as biosorbent. J. Hazard. Mater. 2009, 170, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, E.R. Phenomenological theory of ion solvation effective radii of hydrated ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Heyrovska, R. Dependences of molar volumes in solids, partial molal and hydrated ionic volumes of alkali halides on covalent and ionic radii and the golden ratio. Chem. Phys. Lett. 2007, 436, 287–293. [Google Scholar] [CrossRef]
- Tansel, B.; Sager, J.; Rector, T.; Garland, J.; Strayer, R.F.; Levine, L.; Roberts, M.; Hummerick, M.; Bauer, J. Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep. Purif. Technol. 2006, 51, 40–47. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Omura, A.; Moritomo, Y. Cs+ trapping in size-controlled nanospaces of hexacyanoferrates. Appl. Phys. Express 2012, 5, 057101. [Google Scholar] [CrossRef]
- Yang, M.H.; Jong, S.B.; Lu, C.Y.; Lin, Y.F.; Chiang, P.W.; Tyan, Y.C.; Chung, T.W. Assessing the responses of cellular proteins induced by hyaluronic acid-modified surfaces utilizing a mass spectrometry-based profiling system: Over-Expression of CD36, CD44, CDK9, and PP2A. Analyst 2012, 137, 4921–4933. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Liang, B.; Ogino, A.; Wang, X.K.; Nagatsu, M. Oxygen functionalization of multiwall carbon nanotubes by microwave-excited surface-wave plasma treatment. J. Phys. Chem. C 2009, 113, 7659–7665. [Google Scholar] [CrossRef]
- Saraswati, T.E.; Ogino, A.; Nagatsu, M. Plasma-activated immobilization of biomolecules onto graphite-encapsulated magnetic nanoparticles. Carbon 2012, 50, 1253–1261. [Google Scholar] [CrossRef]
- Shao, D.D.; Ren, X.M.; Hu, J.; Chen, Y.X.; Wang, X.K. Preconcentration of Pb2+ from aqueous solution using poly(acrylamide) and poly(N,N-dimethylacrylamide) grafted multiwalled carbon nanotubes. Colloids Surf. A 2010, 360, 74–84. [Google Scholar] [CrossRef]
- Cao, A.; Xu, C.; Liang, J.; Wu, D.; Wei, B. X-ray diffraction characterization on the alignment degree of carbon nanotubes. Chem. Phys. Lett. 2001, 344, 13–17. [Google Scholar] [CrossRef]
- Yusa, H.; Watanuki, T. X-ray diffraction of multiwalled carbon nanotube under high pressure: Structural durability on static compression. Carbon 2005, 43, 519–523. [Google Scholar] [CrossRef]
- Chen, C.L.; Liang, B.; Lu, D.; Ogino, A.; Wang, X.K.; Nagatsu, M. Amino group introduction onto multiwall carbon nanotubes by NH3/Ar plasma treatment. Carbon 2010, 48, 939–948. [Google Scholar] [CrossRef]
- Lee, A.F.; Baddeley, C.J.; Hardacre, C.; Ormerod, R.M.; Lambert, R.M.; Schmid, G.; West, H. Structural and catalytic properties of novel Au/Pd bimetallic colloid particles: EXAFS, XRD, and acetylene coupling. J. Phys. Chem. 1995, 99, 6096–6102. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Liu, L.; Li, B.; Yang, P.; Zhang, L.; Feng, Y. Adsorption of dye from wastewater using chitosan-CTAB modified bentonites. J. Colloid Interface Sci. 2012, 382, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Burghard, M. Electronic and vibrational properties of chemically modified single-wall carbon nanotubes. Surf. Sci. Rep. 2005, 58, 1–109. [Google Scholar]
- Yang, S.B.; Hu, J.; Chen, C.L.; Shao, D.D.; Wang, X.K. Mutual effects of Pb(II) and humic acid adsorption on multiwalled carbon nanotubes/polyacrylamide composites from aqueous solutions. Environ. Sci. Technol. 2011, 45, 3621–3627. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.G.; Paula, S.; Deamer, D.W. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochem. Bioenerg. 1997, 42, 153–160. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, X.; Wang, Y.; Shi, J. Molecular dynamics study on ionic hydration. Fluid Phase Equilib. 2002, 194–197, 257–270. [Google Scholar] [CrossRef]
- Volkov, A.G. Liquid-liquid interfaces. In Theory and Methods; Deamer, D.W., Ed.; CRC Press: Boca Raton, NY, USA, 1996. [Google Scholar]
- Gourary, B.S.; Adrian, F.J. Wave functions for electron-excess color centers in alkali halide crystals. In Solid State Physics; Frederick, S., David, T., Eds.; Academic Press: Waltham, MA, USA, 1960; pp. 127–247. [Google Scholar]
- O’M, B.J. Review: Ionic hydration in chemistry and biophysics, by B.E. Conway, Elsevier Publishing Company, 1981. J. Solut. Chem. 1982, 11, 221–222. [Google Scholar] [CrossRef]
- Awual, M.R.; Suzuki, S.; Taguchi, T.; Shiwaku, H.; Okamoto, Y.; Yaita, T. Radioactive cesium removal from nuclear wastewater by novel inorganic and conjugate adsorbents. Chem. Eng. J. 2014, 242, 127–135. [Google Scholar] [CrossRef]
- Thammawong, C.; Opaprakasit, P.; Tangboriboonrat, P.; Sreearunothai, P. Prussian blue-coated magnetic nanoparticles for removal of cesium from contaminated environment. J. Nanopart. Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Markham, E.C.; Benton, A.F. The adsorption of gas mixtures by silica. J. Am. Chem. Soc. 1931, 53, 497–507. [Google Scholar] [CrossRef]
- Digiano, F.A.; Baldauf, G.; Frick, B.; Sontheimer, H. A simplified competitive equilibrium adsorption model. Chem. Eng. Sci. 1978, 33, 1667–1673. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Shao, D.; Wang, X.; Hou, G.; Nagatsu, M.; Tan, X.; Ren, X.; Yu, J. Design of Chitosan-Grafted Carbon Nanotubes: Evaluation of How the –OH Functional Group Affects Cs+ Adsorption. Mar. Drugs 2015, 13, 3116-3131. https://doi.org/10.3390/md13053116
Yang S, Shao D, Wang X, Hou G, Nagatsu M, Tan X, Ren X, Yu J. Design of Chitosan-Grafted Carbon Nanotubes: Evaluation of How the –OH Functional Group Affects Cs+ Adsorption. Marine Drugs. 2015; 13(5):3116-3131. https://doi.org/10.3390/md13053116
Chicago/Turabian StyleYang, Shubin, Dadong Shao, Xiangke Wang, Guangshun Hou, Masaaki Nagatsu, Xiaoli Tan, Xuemei Ren, and Jitao Yu. 2015. "Design of Chitosan-Grafted Carbon Nanotubes: Evaluation of How the –OH Functional Group Affects Cs+ Adsorption" Marine Drugs 13, no. 5: 3116-3131. https://doi.org/10.3390/md13053116
APA StyleYang, S., Shao, D., Wang, X., Hou, G., Nagatsu, M., Tan, X., Ren, X., & Yu, J. (2015). Design of Chitosan-Grafted Carbon Nanotubes: Evaluation of How the –OH Functional Group Affects Cs+ Adsorption. Marine Drugs, 13(5), 3116-3131. https://doi.org/10.3390/md13053116